Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation
- PMID: 18796610
- PMCID: PMC2567221
- DOI: 10.1073/pnas.0800589105
Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation
Abstract
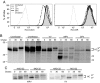
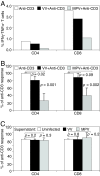
Monkeypox virus (MPV) is a virulent human pathogen that has gained increased attention because of its potential use as a bioterrorism agent and inadvertent introduction into North America in 2003. The US outbreak also provided an important opportunity to study MPV-specific T cell immunity. Although MPV-specific CD4(+) and CD8(+) T cells could recognize vaccinia virus (VV)-infected monocytes and produce inflammatory cytokines such as IFNgamma and TNFalpha, they were largely incapable of responding to autologous MPV-infected cells. Further analysis revealed that, unlike cowpox virus (CPV), MPV did not interfere with MHC expression or intracellular transport of MHC molecules. Instead, MPV-infected cells were capable of preventing T cell receptor (TcR)-mediated T cell activation in trans. The ability to trigger a state of nonresponsiveness represents a unique MHC-independent mechanism for blocking antiviral T cell activation and inflammatory cytokine production and is likely an important attribute involved with viral dissemination in the infected host.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge.Virology. 2007 Sep 15;366(1):73-83. doi: 10.1016/j.virol.2007.04.010. Epub 2007 May 16. Virology. 2007. PMID: 17507071 Free PMC article.
-
Cowpox virus evades CTL recognition and inhibits the intracellular transport of MHC class I molecules.J Immunol. 2007 Feb 1;178(3):1654-61. doi: 10.4049/jimmunol.178.3.1654. J Immunol. 2007. PMID: 17237415
-
Epstein-Barr virus evasion of CD8(+) and CD4(+) T cell immunity via concerted actions of multiple gene products.Semin Cancer Biol. 2008 Dec;18(6):397-408. doi: 10.1016/j.semcancer.2008.10.008. Epub 2008 Oct 25. Semin Cancer Biol. 2008. PMID: 18977445 Review.
-
Comparison of host cell gene expression in cowpox, monkeypox or vaccinia virus-infected cells reveals virus-specific regulation of immune response genes.Virol J. 2013 Feb 20;10:61. doi: 10.1186/1743-422X-10-61. Virol J. 2013. PMID: 23425254 Free PMC article.
-
Mechanisms of immune evasion of monkeypox virus.Front Microbiol. 2023 Feb 1;14:1106247. doi: 10.3389/fmicb.2023.1106247. eCollection 2023. Front Microbiol. 2023. PMID: 36819041 Free PMC article. Review.
Cited by
-
The Re-Emergence of Mpox: Old Illness, Modern Challenges.Biomedicines. 2024 Jul 1;12(7):1457. doi: 10.3390/biomedicines12071457. Biomedicines. 2024. PMID: 39062032 Free PMC article. Review.
-
Clinical Manifestation, Transmission, Pathogenesis, and Diagnosis of Monkeypox Virus: A Comprehensive Review.Life (Basel). 2023 Feb 14;13(2):522. doi: 10.3390/life13020522. Life (Basel). 2023. PMID: 36836879 Free PMC article. Review.
-
Monkeypox in a patient with HIV: case report.Rev Peru Med Exp Salud Publica. 2023 Apr-Jun;40(2):229-235. doi: 10.17843/rpmesp.2023.402.12344. Rev Peru Med Exp Salud Publica. 2023. PMID: 38232270 Free PMC article.
-
Orthopoxvirus-Specific T-Cell Responses in Convalescent Mpox Patients.J Infect Dis. 2024 Jan 12;229(1):54-58. doi: 10.1093/infdis/jiad245. J Infect Dis. 2024. PMID: 37380166 Free PMC article.
-
Monkeypox, a Literature Review: What Is New and Where Does This concerning Virus Come From?Viruses. 2022 Aug 27;14(9):1894. doi: 10.3390/v14091894. Viruses. 2022. PMID: 36146705 Free PMC article. Review.
References
-
- Henderson DA. The looming threat of bioterrorism. Science. 1999;283:1279–1282. - PubMed
-
- O'Toole T, Mair M, Inglesby TV. Shining light on “Dark Winter.”. Clin Infect Dis. 2002;34:972–983. - PubMed
-
- Smith GL, McFadden G. Smallpox: Anything to declare? Nat Rev Immunol. 2002;2:521–527. - PubMed
-
- Jezek Z, Szczeniowski M, Paluku KM, Mutombo M. Human monkeypox: Clinical features of 282 patients. J Infect Dis. 1987;156:293–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

