NMR structures of two designed proteins with high sequence identity but different fold and function
- PMID: 18796611
- PMCID: PMC2567172
- DOI: 10.1073/pnas.0805857105
NMR structures of two designed proteins with high sequence identity but different fold and function
Abstract
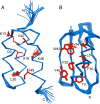
How protein sequence codes for 3D structure remains a fundamental question in biology. One approach to understanding the folding code is to design a pair of proteins with maximal sequence identity but retaining different folds. Therefore, the nonidentities must be responsible for determining which fold topology prevails and constitute a fold-specific folding code. We recently designed two proteins, G(A)88 and G(B)88, with 88% sequence identity but different folds and functions [Alexander et al. (2007) Proc Natl Acad Sci USA 104:11963-11968]. Here, we describe the detailed 3D structures of these proteins determined in solution by NMR spectroscopy. Despite a large number of mutations taking the sequence identity level from 16 to 88%, G(A)88 and G(B)88 maintain their distinct wild-type 3-alpha and alpha/beta folds, respectively. To our knowledge, the 3D-structure determination of two monomeric proteins with such high sequence identity but different fold topology is unprecedented. The geometries of the seven nonidentical residues (of 56 total) provide insights into the structural basis for switching between 3-alpha and alpha/beta conformations. Further mutation of a subset of these nonidentities, guided by the G(A)88 and G(B)88 structures, leads to proteins with even higher levels of sequence identity (95%) and different folds. Thus, conformational switching to an alternative monomeric fold of comparable stability can be effected with just a handful of mutations in a small protein. This result has implications for understanding not only the folding code but also the evolution of new folds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–230. - PubMed
-
- Cordes MH, Walsh NP, McKnight CJ, Sauer RT. Evolution of a protein fold in vitro. Science. 1999;284:325–328. - PubMed
-
- Rose GD, Creamer TP. Protein folding: Predicting predicting. Proteins. 1994;19:1–3. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

