Incomplete restoration of Mpl expression in the mpl-/- mouse produces partial correction of the stem cell-repopulating defect and paradoxical thrombocytosis
- PMID: 18796624
- PMCID: PMC2647669
- DOI: 10.1182/blood-2007-11-124859
Incomplete restoration of Mpl expression in the mpl-/- mouse produces partial correction of the stem cell-repopulating defect and paradoxical thrombocytosis
Abstract
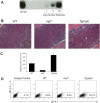
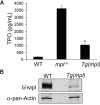
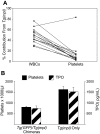
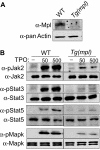
Expression of Mpl is restricted to hematopoietic cells in the megakaryocyte lineage and to undifferentiated progenitors, where it initiates critical cell survival and proliferation signals after stimulation by its ligand, thrombopoietin (TPO). As a result, a deficiency in Mpl function in patients with congenital amegakaryocytic thrombocytopenia (CAMT) and in mpl(-/-) mice produces profound thrombocytopenia and a severe stem cell-repopulating defect. Gene therapy has the potential to correct the hematopoietic defects of CAMT by ectopic gene expression that restores normal Mpl receptor activity. We rescued the mpl(-/-) mouse with a transgenic vector expressing mpl from the promoter elements of the 2-kb region of DNA just proximal to the natural gene start site. Transgene rescued mice exhibit thrombocytosis but only partial correction of the stem cell defect. Furthermore, they show very low-level expression of Mpl on platelets and megakaryocytes, and the transgene-rescued megakaryocytes exhibit diminished TPO-dependent kinase phosphorylation and reduced platelet production in bone marrow chimeras. Thrombocytosis is an unexpected consequence of reduced Mpl expression and activity. However, impaired TPO homeostasis in the transgene-rescued mice produces elevated plasma TPO levels, which serves as an unchecked stimulus to drive the observed excessive megakaryocytopoiesis.
Figures


Comment in
-
Mpl and thrombocytosis: levels matter.Blood. 2009 Feb 19;113(8):1617-8. doi: 10.1182/blood-2008-11-186981. Blood. 2009. PMID: 19228929 No abstract available.
References
-
- Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034–2045. - PubMed
-
- Gurney AL, Carver-Moore K, de Sauvage FJ, Moore MW. Thrombocytopenia in c-mpl-deficient mice. Science. 1994;265:1445–1447. - PubMed
-
- Antonchuk J, Hyland CD, Hilton DJ, Alexander WS. Synergistic effects on erythropoiesis, thrombopoiesis, and stem cell competitiveness in mice deficient in thrombopoietin and steel factor receptors. Blood. 2004;104:1306–1313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

