Nonresectable hepatocellular carcinoma: long-term toxicity in patients treated with transarterial chemoembolization--single-center experience
- PMID: 18796686
- PMCID: PMC6944073
- DOI: 10.1148/radiol.2483071902
Nonresectable hepatocellular carcinoma: long-term toxicity in patients treated with transarterial chemoembolization--single-center experience
Abstract
Purpose: To determine the toxicity profile of transarterial chemoembolization (TACE) at 6 months and 1 year after treatment in patients with hepatocellular carcinoma (HCC) in a standardized oncology protocol so that TACE could be compared with systemic chemotherapeutic regimens for liver cancer.
Materials and methods: The study was authorized by the institutional review board. Between January 2002 and January 2007, 190 patients (155 men, 35 women; median age, 65 years; age range, 18-84 years) with HCC who underwent TACE treatment were identified from a prospectively collected database. Clinical records of complete blood cell counts and chemical profiles at baseline and at 6 and 12 months after treatment were studied retrospectively. Toxicity was graded according to the common terminology criteria for adverse events (CTCAE). A transition (survival) analysis perspective was used to estimate the distribution of toxicity grades. Patient survival from the first TACE session was calculated with Kaplan-Meier analysis.
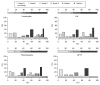
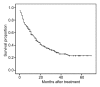
Results: Grade 3 or 4 toxicity 6 and 12 months, respectively, after treatment included leukocytopenia (7% and 19%); anemia (9% and 19%); thromobocytopenia (13% and 23%); prolonged activated partial thromboplastin time (8% and 18%); elevated aspartate aminotransferase (15% and 18%), alanine aminotransferase (10% and 18%), and alkaline phosphatase (8% and 18%) levels; hypoalbuminemia (10% and 19%); hyperbilirubinemia (10% and 22%); and alopecia (18%). The cumulative survival rate was 58% at 1 year, 39% at 2 years, and 29% at 3 years. These toxicity rates were considerably lower than those reported after treatment with currently used systemic chemotherapeutic agents.
Conclusion: Study results show that TACE has a favorable long-term toxicity profile in patients with HCC. Data clearly support the role of TACE in the treatment of patients with nonresectable HCC.
(c) RSNA, 2008.
Figures

Similar articles
-
Efficacy of transarterial chemoembolization targeting portal vein tumor thrombus in patients with hepatocellular carcinoma.Anticancer Res. 2014 Aug;34(8):4231-7. Anticancer Res. 2014. PMID: 25075052
-
Transarterial chemoembolization with cisplatin as second-line treatment for hepatocellular carcinoma unresponsive to chemoembolization with epirubicin-Lipiodol emulsion.Cardiovasc Intervent Radiol. 2012 Feb;35(1):82-9. doi: 10.1007/s00270-010-0086-6. Epub 2011 Jan 4. Cardiovasc Intervent Radiol. 2012. PMID: 21203761
-
Morbidity and mortality following transarterial liver chemoembolization in patients with hepatocellular carcinoma and synthetic hepatic dysfunction.Liver Transpl. 2013 Feb;19(2):164-73. doi: 10.1002/lt.23552. Epub 2012 Dec 12. Liver Transpl. 2013. PMID: 23008091
-
Prognostic predictors for patients with hepatocellular carcinoma receiving adjuvant transcatheter arterial chemoembolization.Eur J Gastroenterol Hepatol. 2019 Jul;31(7):836-844. doi: 10.1097/MEG.0000000000001346. Eur J Gastroenterol Hepatol. 2019. PMID: 30614882
-
Patients with unresectable hepatocellular carcinoma beyond Milan criteria: should we perform transarterial chemoembolization or liver transplantation?Transplant Proc. 2010 Apr;42(3):821-4. doi: 10.1016/j.transproceed.2010.02.027. Transplant Proc. 2010. PMID: 20430181
Cited by
-
Bringing the heavy: carbon ion therapy in the radiobiological and clinical context.Radiat Oncol. 2014 Mar 28;9(1):88. doi: 10.1186/1748-717X-9-88. Radiat Oncol. 2014. PMID: 24679134 Free PMC article. Review.
-
Clinical impact of selective transarterial chemoembolization on hepatocellular carcinoma: a cohort study.World J Gastroenterol. 2009 Apr 21;15(15):1843-8. doi: 10.3748/wjg.15.1843. World J Gastroenterol. 2009. PMID: 19370781 Free PMC article.
-
Identification of predictive factors for post-transarterial chemoembolization liver failure in hepatocellular carcinoma patients: A retrospective study.World J Clin Cases. 2022 Aug 26;10(24):8535-8546. doi: 10.12998/wjcc.v10.i24.8535. World J Clin Cases. 2022. PMID: 36157824 Free PMC article.
-
Systematic review of catheter-based intra-arterial therapies in hepatocellular carcinoma: state of the art and future directions.Br J Radiol. 2015 Aug;88(1052):20140564. doi: 10.1259/bjr.20140564. Epub 2015 May 15. Br J Radiol. 2015. PMID: 25978585 Free PMC article.
-
Tumor uptake of hollow gold nanospheres after intravenous and intra-arterial injection: PET/CT study in a rabbit VX2 liver cancer model.Mol Imaging Biol. 2013 Oct;15(5):614-24. doi: 10.1007/s11307-013-0635-x. Mol Imaging Biol. 2013. PMID: 23608932 Free PMC article.
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001;94:153–156. - PubMed
-
- El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999;340:745–750. - PubMed
-
- McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev 2006;15:1198–1203. - PubMed
-
- Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 2002;35:519–524. - PubMed
-
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

