Histidine-rich glycoprotein protects from systemic Candida infection
- PMID: 18797515
- PMCID: PMC2537934
- DOI: 10.1371/journal.ppat.1000116
Histidine-rich glycoprotein protects from systemic Candida infection
Abstract
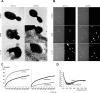
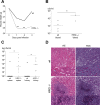
Fungi, such as Candida spp., are commonly found on the skin and at mucosal surfaces. Yet, they rarely cause invasive infections in immunocompetent individuals, an observation reflecting the ability of our innate immune system to control potentially invasive microbes found at biological boundaries. Antimicrobial proteins and peptides are becoming increasingly recognized as important effectors of innate immunity. This is illustrated further by the present investigation, demonstrating a novel antifungal role of histidine-rich glycoprotein (HRG), an abundant and multimodular plasma protein. HRG bound to Candida cells, and induced breaks in the cell walls of the organisms. Correspondingly, HRG preferentially lysed ergosterol-containing liposomes but not cholesterol-containing ones, indicating a specificity for fungal versus other types of eukaryotic membranes. Both antifungal and membrane-rupturing activities of HRG were enhanced at low pH, and mapped to the histidine-rich region of the protein. Ex vivo, HRG-containing plasma as well as fibrin clots exerted antifungal effects. In vivo, Hrg(-/-) mice were susceptible to infection by C. albicans, in contrast to wild-type mice, which were highly resistant to infection. The results demonstrate a key and previously unknown antifungal role of HRG in innate immunity.
Conflict of interest statement
Drs. Schmidtchen and Malmsten have shares in DermaGen AB, a company involved in therapeutical development of antimicrobial peptides. Peptides of HRG are included in patent applications of the company.
Figures
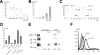



Similar articles
-
Phagocytes from Mice Lacking the Sts Phosphatases Have an Enhanced Antifungal Response to Candida albicans.mBio. 2018 Jul 17;9(4):e00782-18. doi: 10.1128/mBio.00782-18. mBio. 2018. PMID: 30018105 Free PMC article.
-
Histidine-rich glycoprotein promotes bacterial entrapment in clots and decreases mortality in a mouse model of sepsis.Blood. 2010 Sep 30;116(13):2365-72. doi: 10.1182/blood-2010-02-271858. Epub 2010 Jun 29. Blood. 2010. PMID: 20587784
-
Histidine-Rich Glycoprotein Inhibits HIV-1 Infection in a pH-Dependent Manner.J Virol. 2019 Feb 5;93(4):e01749-18. doi: 10.1128/JVI.01749-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30518643 Free PMC article.
-
New insights into innate immune control of systemic candidiasis.Med Mycol. 2014 Aug;52(6):555-64. doi: 10.1093/mmy/myu029. Epub 2014 Jul 14. Med Mycol. 2014. PMID: 25023483 Free PMC article. Review.
-
Immunity to Candida.Oral Dis. 2002;8 Suppl 2:69-75. doi: 10.1034/j.1601-0825.2002.00015.x. Oral Dis. 2002. PMID: 12164664 Review.
Cited by
-
Longitudinal Measurement of Histidine-Rich Glycoprotein Levels in Bronchopulmonary Dysplasia: A Pilot Study.Biomedicines. 2023 Jan 14;11(1):212. doi: 10.3390/biomedicines11010212. Biomedicines. 2023. PMID: 36672720 Free PMC article.
-
Histidine-Rich Glycoprotein Stimulates Human Neutrophil Phagocytosis and Prolongs Survival through CLEC1A.J Immunol. 2021 Feb 15;206(4):737-750. doi: 10.4049/jimmunol.2000817. Epub 2021 Jan 15. J Immunol. 2021. PMID: 33452125 Free PMC article.
-
Flor Yeast: New Perspectives Beyond Wine Aging.Front Microbiol. 2016 Apr 14;7:503. doi: 10.3389/fmicb.2016.00503. eCollection 2016. Front Microbiol. 2016. PMID: 27148192 Free PMC article. Review.
-
Proteogenomic Features of the Highly Polymorphic Histidine-rich Glycoprotein Arose Late in Evolution.Mol Cell Proteomics. 2023 Jul;22(7):100585. doi: 10.1016/j.mcpro.2023.100585. Epub 2023 May 25. Mol Cell Proteomics. 2023. PMID: 37244517 Free PMC article.
-
Proteolysis of human thrombin generates novel host defense peptides.PLoS Pathog. 2010 Apr 22;6(4):e1000857. doi: 10.1371/journal.ppat.1000857. PLoS Pathog. 2010. PMID: 20421939 Free PMC article.
References
-
- Lehrer RI, Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Curr Opin Hematol. 2002;9:18–22. - PubMed
-
- Harder J, Glaser R, Schröder JM. Review: Human antimicrobial proteins effectors of innate immunity. J Endotoxin Res. 2007;13:317–338. - PubMed
-
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. - PubMed
-
- Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

