A role for planar cell polarity signaling in angiogenesis
- PMID: 18798004
- PMCID: PMC3547076
- DOI: 10.1007/s10456-008-9116-2
A role for planar cell polarity signaling in angiogenesis
Abstract
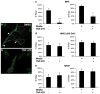
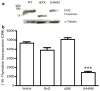
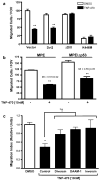
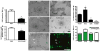
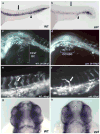
The planar cell polarity (PCP) pathway is a highly conserved signaling cascade that coordinates both epithelial and axonal morphogenic movements during development. Angiogenesis also involves the growth and migration of polarized cells, although the mechanisms underlying their intercellular communication are poorly understood. Here, using cell culture assays, we demonstrate that inhibition of PCP signaling disrupts endothelial cell growth, polarity, and migration, all of which can be rescued through downstream activation of this pathway by expression of either Daam-1, Diversin or Inversin. Silencing of either Dvl2 or Prickle suppressed endothelial cell proliferation. Moreover, loss of p53 rescues endothelial cell growth arrest but not the migration inhibition caused by PCP disruption. In addition, we show that the zebrafish Wnt5 mutant (pipetail (ppt)), which has impaired PCP signaling, displays vascular developmental defects. These findings reveal a potential role for PCP signaling in the coordinated assembly of endothelial cells into vascular structures and have important implications for vascular remodeling in development and disease.
Conflict of interest statement
The authors have declared no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

