Human papillomavirus infection and the primary and secondary prevention of cervical cancer
- PMID: 18798536
- PMCID: PMC6263938
- DOI: 10.1002/cncr.23704
Human papillomavirus infection and the primary and secondary prevention of cervical cancer
Abstract
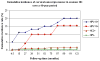
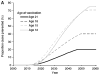
A wealth of evidence has led to the conclusion that virtually all cases of cervical cancer are attributable to persistent infection by a subset of human papillomavirus (HPV) types, especially HPV type 16 (HPV-16) and HPV-18. These HPV types also cause a proportion of other cancers, including vulvar, vaginal, anal, penile, and oropharyngeal cancers. Although cervical cancer screening, primarily with the Papanicolaou (Pap) smear, has reduced the incidence of this cancer in industrialized countries, cervical cancer remains the second most common cause of death from cancer in women worldwide, because the developing world has lacked the resources for widespread, high-quality screening. In addition to advances in Pap smear technology, the identification of HPV as the etiologic agent has produced 2 recent advances that may have a major impact on approaches to reduce the incidence of this disease. The first is the development of a preventive vaccine, the current versions of which appear to prevent close to 100% of persistent genital infection and disease caused by HPV-16 and HPV-18; future second-generation vaccines may be able to protect against oncogenic infections by a broader array of HPV types. The second is the incorporation of HPV testing into screening programs. In women aged >30 years, HPV testing can identify high-grade cervical intraepithelial neoplasia earlier than Pap smears with acceptable rates of specificity. These results, together with the high sensitivity of HPV testing, suggest that such testing could permit increased intervals for screening. An inexpensive HPV test in development, if successful, may be incorporated as part of an economically viable 'screen-and-treat' approach in the developing world. The manner in which vaccination and screening programs are integrated will need to be considered carefully so that they are efficient in reducing the overall incidence of cervical cancer.
Figures
References
-
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030–44. - PubMed
-
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324(1):17–27. - PubMed
-
- Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111(2):278–85. - PubMed
-
- Schiffman M, Herrero R, Desalle R, Hildesheim A, Wacholder S, Rodriguez AC, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337(1):76–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

