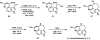
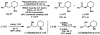
Pd-catalyzed enantioselective aerobic oxidation of secondary alcohols: applications to the total synthesis of alkaloids
- PMID: 18798630
- PMCID: PMC2574996
- DOI: 10.1021/ja804738b
Pd-catalyzed enantioselective aerobic oxidation of secondary alcohols: applications to the total synthesis of alkaloids
Abstract
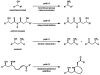
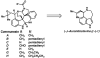
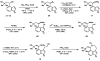
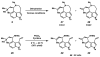
Enantioselective syntheses of the alkaloids (-)-aurantioclavine, (+)-amurensinine, (-)-lobeline, and (-)- and (+)-sedamine are described. The syntheses demonstrate the effectiveness of the Pd-catalyzed asymmetric oxidation of secondary alcohols in diverse contexts and the ability of this methodology to set the absolute configuration of multiple stereocenters in a single operation. The utility of an aryne C-C insertion reaction in accessing complex polycyclic frameworks is also described.
Figures
References
-
-
For recent reviews on the enantioselective synthesis of amines, see: Moody CJ. Angew. Chem., Int. Ed. 2007;46:9148.Ouellet SG, Walji AM, MacMillan DWC. Acc. Chem. Res. 2007;40:1327.Skucas E, Ngai M-Y, Komanduri V, Krische MJ. Acc. Chem. Res. 2007;40:1394.Masson G, Housseman C, Zhu J. Angew. Chem., Int. Ed. 2007;46:4614.Li G, Saibau K, Timmons C. Eur. J. Org. Chem. 2007;17:2745.Bräse S, Baumann T, Dahmen S, Vogt H. Chem. Commun. 2007:1881.Maruoka K, Ooi T, Kano T. Chem. Commun. 2007:1487.Ellman JA. Pure Appl. Chem. 2003;75:39.Roesky PW, Mueller TE. Angew. Chem., Int. Ed. 2003;42:2708.Enders D, Reinhold U. Tetrahedron: Asymmetry. 1997;8:1895.
-
-
-
For selected examples of synthesis of amines directly from alcohols, their corresponding esters, carbonates, sulfonate esters or alkyl halides, see: Kan T, Fukuyama T. Chem. Commun. 2004:353.Fujita K-i., Fujii T, Yamaguchi R. Org. Lett. 2004;6:3525.Evans PA, Robinson JE, Nelson JD. J. Am. Chem. Soc. 1999;121:6761.Scriven EFV, Turnbull K. Chem. Rev. 1988;88:297.Kolasa T, Miller MJ. J. Org. Chem. 1987;52:4978.Bestmann HJ, Wölfel G. Chem. Ber. 1984;117:1250.Trost BM, Keinan E. J. Org. Chem. 1979;44:3451.Nordlander JE, Catalane DB, Eberlein TH, Farkas LV, Howe RS, Stevens RM, Tripoulas NA, Stansfield RE, Cox JL. Tetrahedron Lett. 1978;50:4987.Jonczyk A, Ochal Z, Makosza M. Synthesis. 1978:882.Zwierzak A, Podstawczynska I. Angew. Chem., Int. Ed. Engl. 1977;16:702.Hendrickson JB, Joffee I. J. Am. Chem. Soc. 1973;95:4083.Mitsunobu O, Wada M, Sano T. J. Am. Chem. Soc. 1972;94:679.Gibson MS, Bradshaw RW. Angew. Chem., Int. Ed. Engl. 1968;7:919.
-
-
-
For recent reviews of asymmetric desymmetrization, see: Rendler S, Ostreich M. Angew. Chem., Int. Ed. 2008;47:248.Rovis T. Recent Advances in Catalytic Asymmetric Desymmetrization Reactions. In: Mikami K, Lautens M, editors. New Frontiers in Asymmetric Catalysis. John Wiley & Sons, Inc.; Hoboken, NJ: 2007. pp. 275–311.Atodiresei I, Schiffers I, Bolm C. Chem. Rev. 2007;107:5683.Schneider C. Synthesis. 2006;23:3919.Garcia-Urdiales E, Alfonso I, Gotor V. Chem. Rev. 2005;105:313.
-
-
-
For an experimentally derived model for stereoselectivity in the oxidative kinetic resolution of benzylic alcohols catalyzed by Pd(sparteine)Cl2, see Trend RM, Stoltz BM. J. Am. Chem. Soc. 2004;126:4482.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources