Platelets, petechiae, and preservation of the vascular wall
- PMID: 18799560
- PMCID: PMC2935201
- DOI: 10.1056/NEJMra0800887
Platelets, petechiae, and preservation of the vascular wall
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
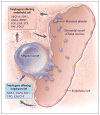
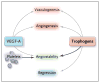


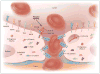
Comment in
-
Platelets and the vascular wall.N Engl J Med. 2008 Dec 18;359(25):2727-8; author reply 2728. doi: 10.1056/NEJMc082157. N Engl J Med. 2008. PMID: 19092160 No abstract available.
References
-
- Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HL066952/HL/NHLBI NIH HHS/United States
- R01 HL058707/HL/NHLBI NIH HHS/United States
- P50 HL084936/HL/NHLBI NIH HHS/United States
- P01 HL072942/HL/NHLBI NIH HHS/United States
- P01 HL067839/HL/NHLBI NIH HHS/United States
- RC1 AI080309/AI/NIAID NIH HHS/United States
- R01 HL075234/HL/NHLBI NIH HHS/United States
- R21 HL083222/HL/NHLBI NIH HHS/United States
- R01 HL097797/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P01 HL059312/HL/NHLBI NIH HHS/United States
- R01 HL061849/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
