Retrograde flow and myosin II activity within the leading cell edge deliver F-actin to the lamella to seed the formation of graded polarity actomyosin II filament bundles in migrating fibroblasts
- PMID: 18799629
- PMCID: PMC2575164
- DOI: 10.1091/mbc.e08-01-0034
Retrograde flow and myosin II activity within the leading cell edge deliver F-actin to the lamella to seed the formation of graded polarity actomyosin II filament bundles in migrating fibroblasts
Abstract
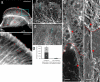
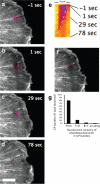
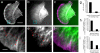
In migrating fibroblasts actomyosin II bundles are graded polarity (GP) bundles, a distinct organization to stress fibers. GP bundles are important for powering cell migration, yet have an unknown mechanism of formation. Electron microscopy and the fate of photobleached marks show actin filaments undergoing retrograde flow in filopodia, and the lamellipodium are structurally and dynamically linked with stationary GP bundles within the lamella. An individual filopodium initially protrudes, but then becomes separated from the tip of the lamellipodium and seeds the formation of a new GP bundle within the lamella. In individual live cells expressing both GFP-myosin II and RFP-actin, myosin II puncta localize to the base of an individual filopodium an average 28 s before the filopodium seeds the formation of a new GP bundle. Associated myosin II is stationary with respect to the substratum in new GP bundles. Inhibition of myosin II motor activity in live cells blocks appearance of new GP bundles in the lamella, without inhibition of cell protrusion in the same timescale. We conclude retrograde F-actin flow and myosin II activity within the leading cell edge delivers F-actin to the lamella to seed the formation of new GP bundles.
Figures



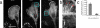
References
-
- Badley R. A., Couchman J. R., Rees D. A. Comparison of the cell cytoskeleton in migratory and stationary chick fibroblasts. J. Muscle Res. Cell Motil. 1980;1:5–14. - PubMed
-
- Bernstein B. W., Bamburg J. R. A proposed mechanism for cell polarization with no external cues. Cell Motil. Cytoskelet. 2004;58:96–103. - PubMed
-
- Burgess D. R. Cytokinesis: new roles for myosin. Curr. Biol. 2005;15:R310–R311. - PubMed
-
- Carlier M. F., Pantaloni D. Control of actin assembly dynamics in cell motility. J. Biol. Chem. 2007;282:23005–23009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

