Activation of Na+/H+ and K+/H+ exchange by calyculin A in Amphiuma tridactylum red blood cells: implications for the control of volume-induced ion flux activity
- PMID: 18799654
- PMCID: PMC2584995
- DOI: 10.1152/ajpcell.00160.2008
Activation of Na+/H+ and K+/H+ exchange by calyculin A in Amphiuma tridactylum red blood cells: implications for the control of volume-induced ion flux activity
Abstract
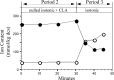
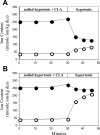
Alteration in cell volume of vertebrates results in activation of volume-sensitive ion flux pathways. Fine control of the activity of these pathways enables cells to regulate volume following osmotic perturbation. Protein phosphorylation and dephosphorylation have been reported to play a crucial role in the control of volume-sensitive ion flux pathways. Exposing Amphiuma tridactylu red blood cells (RBCs) to phorbol esters in isotonic medium results in a simultaneous, dose-dependent activation of both Na(+)/H(+) and K(+)/H(+) exchangers. We tested the hypothesis that in Amphiuma RBCs, both shrinkage-induced Na(+)/H(+) exchange and swelling-induced K(+)/H(+) exchange are activated by phosphorylation-dependent reactions. To this end, we assessed the effect of calyculin A, a phosphatase inhibitor, on the activity of the aforementioned exchangers. We found that exposure of Amphiuma RBCs to calyculin-A in isotonic media results in simultaneous, 1-2 orders of magnitude increase in the activity of both K(+)/H(+) and Na(+)/H(+) exchangers. We also demonstrate that, in isotonic media, calyculin A-dependent increases in net Na(+) uptake and K(+) loss are a direct result of phosphatase inhibition and are not dependent on changes in cell volume. Whereas calyculin A exposure in the absence of volume changes results in stimulation of both the Na(+)/H(+) and K(+)/H(+) exchangers, superimposing cell swelling or shrinkage and calyculin A treatment results in selective activation of K(+)/H(+) or Na(+)/H(+) exchange, respectively. We conclude that kinase-dependent reactions are responsible for Na(+)/H(+) and K(+)/H(+) exchange activity, whereas undefined volume-dependent reactions confer specificity and coordinated control.
Figures







Similar articles
-
Coordinated control of volume regulatory Na+/H+ and K+/H+ exchange pathways in Amphiuma red blood cells.Am J Physiol Cell Physiol. 2010 Mar;298(3):C510-20. doi: 10.1152/ajpcell.00141.2009. Epub 2009 Nov 25. Am J Physiol Cell Physiol. 2010. PMID: 19940069 Free PMC article.
-
Activation of sodium transport in rat erythrocytes by inhibition of protein phosphatases 1 and 2A.Comp Biochem Physiol B Biochem Mol Biol. 2006 Sep;145(1):60-7. doi: 10.1016/j.cbpb.2006.06.005. Epub 2006 Jun 21. Comp Biochem Physiol B Biochem Mol Biol. 2006. PMID: 16875859
-
Cell volume regulation by Amphiuma red blood cells. The role of Ca+2 as a modulator of alkali metal/H+ exchange.J Gen Physiol. 1983 Dec;82(6):761-84. doi: 10.1085/jgp.82.6.761. J Gen Physiol. 1983. PMID: 6420507 Free PMC article.
-
Cell volume and pH regulation by the Amphiuma red blood cell: a model for hypoxia-induced cell injury.Comp Biochem Physiol Comp Physiol. 1992 Aug;102(4):603-8. doi: 10.1016/0300-9629(92)90711-x. Comp Biochem Physiol Comp Physiol. 1992. PMID: 1355022 Review.
-
Regulation of Na+/H+ antiporter in trout red blood cells.J Exp Biol. 1997 Jan;200(Pt 2):353-60. doi: 10.1242/jeb.200.2.353. J Exp Biol. 1997. PMID: 9050244 Review.
Cited by
-
Activation of Na+/H+ exchange by protein phosphatase inhibitors in red blood cells of the frog Rana ridibunda.J Comp Physiol B. 2003 Jul;173(5):429-35. doi: 10.1007/s00360-003-0351-y. Epub 2003 May 20. J Comp Physiol B. 2003. PMID: 12756484
-
Phosphorylation and activation of the plasma membrane Na+/H+ exchanger (NHE1) during osmotic cell shrinkage.PLoS One. 2011;6(12):e29210. doi: 10.1371/journal.pone.0029210. Epub 2011 Dec 28. PLoS One. 2011. PMID: 22216214 Free PMC article.
-
Coordinated control of volume regulatory Na+/H+ and K+/H+ exchange pathways in Amphiuma red blood cells.Am J Physiol Cell Physiol. 2010 Mar;298(3):C510-20. doi: 10.1152/ajpcell.00141.2009. Epub 2009 Nov 25. Am J Physiol Cell Physiol. 2010. PMID: 19940069 Free PMC article.
References
-
- Aharonovitz O, Demaurex N, Woodside M, Grinstein S. ATP dependence is not an intrinsic property of Na+/H+ exchanger NHE1: requirement for an ancillary factor. Am J Physiol Cell Physiol 276: C1303–C1311, 1999. - PubMed
-
- Bertrand B, Wakabayashi S, Ikeda T, Pouysségur J, Shigekawa M. The Na+/H+ exchanger isoform 1 (NHE1) is a novel member of the calmodulin-binding proteins. Identification and characterization of calmodulin-binding sites. J Biol Chem 269: 13703–13709, 1994. - PubMed
-
- Bianchini L, Woodside M, Sardet C, Pouyssegur J, Takai A, Grinstein S. Okadaic acid, a phosphatase inhibitor, induces activation and phosphorylation of the Na+/H+ antiport. J Biol Chem 266: 15406–15413, 1991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

