Metabolic environment in substantia nigra reticulata is critical for the expression and control of hypoglycemia-induced seizures
- PMID: 18799669
- PMCID: PMC2615494
- DOI: 10.1523/JNEUROSCI.3195-08.2008
Metabolic environment in substantia nigra reticulata is critical for the expression and control of hypoglycemia-induced seizures
Abstract
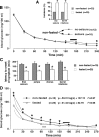
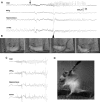
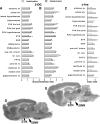
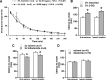
Seizures represent a common and serious complication of hypoglycemia. Here we studied mechanisms of control of hypoglycemic seizures induced by insulin injection in fasted and nonfasted rats. We demonstrate that fasting predisposes rats to more rapid and consistent development of hypoglycemic seizures. However, the fasting-induced decrease in baseline blood glucose concentration cannot account for the earlier onset of seizures in fasted versus nonfasted rats. Data obtained with c-Fos immunohistochemistry and [14C]2-deoxyglucose uptake implicate a prominent involvement of the substantia nigra reticulata (SNR) among other structures in the hypoglycemic seizure control. This is supported by data showing that fasting decreases the SNR expression of K(ATP) channels, which link metabolism with activity, and is further confirmed with microinfusions of K(ATP) channel agonist and antagonist. Data obtained with whole-cell and perforated patch recordings from SNR neurons in slices in vitro demonstrate that both presynaptic and postsynaptic K(ATP) channels participate in the failure of the SNR to control hypoglycemic seizures. The results suggest that fasting and insulin-induced hypoglycemia can lead to impairment in the function of the SNR, leading thus to hypoglycemic seizures.
Figures



Similar articles
-
The involvement of the substantia nigra pars reticulata in hypoglycemic seizures.Epilepsia. 2007;48 Suppl 5:106-8. doi: 10.1111/j.1528-1167.2007.01296.x. Epilepsia. 2007. PMID: 17910588
-
Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure.Science. 2001 May 25;292(5521):1543-6. doi: 10.1126/science.1059829. Science. 2001. PMID: 11375491
-
Effects of barbiturates on ATP-sensitive K channels in rat substantia nigra.Neuroscience. 2006;137(2):573-81. doi: 10.1016/j.neuroscience.2005.08.078. Epub 2005 Nov 14. Neuroscience. 2006. PMID: 16289884
-
Ontogeny and topography of seizure regulation by the substantia nigra.Brain Dev. 1995;17 Suppl:61-72. doi: 10.1016/0387-7604(95)90074-8. Brain Dev. 1995. PMID: 8882575 Review.
-
Neuroprotection by KATP channels.J Mol Cell Cardiol. 2005 Jun;38(6):945-9. doi: 10.1016/j.yjmcc.2004.11.020. Epub 2005 Feb 5. J Mol Cell Cardiol. 2005. PMID: 15910879 Review.
Cited by
-
Developmental and sex differences in tetramethylenedisulfotetramine (TMDT)-induced syndrome in rats.Dev Neurobiol. 2018 Apr;78(4):403-416. doi: 10.1002/dneu.22582. Epub 2018 Feb 14. Dev Neurobiol. 2018. PMID: 29411537 Free PMC article.
-
Severe hypoglycemia-induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation.Diabetes. 2013 Oct;62(10):3570-81. doi: 10.2337/db13-0216. Epub 2013 Jul 8. Diabetes. 2013. PMID: 23835337 Free PMC article.
-
Severe hypoglycemia in a juvenile diabetic rat model: presence and severity of seizures are associated with mortality.PLoS One. 2013 Dec 30;8(12):e83168. doi: 10.1371/journal.pone.0083168. eCollection 2013. PLoS One. 2013. PMID: 24386156 Free PMC article.
-
Dopamine D1 Receptor Immunoreactivity on Fine Processes of GFAP-Positive Astrocytes in the Substantia Nigra Pars Reticulata of Adult Mouse.Front Neuroanat. 2017 Feb 1;11:3. doi: 10.3389/fnana.2017.00003. eCollection 2017. Front Neuroanat. 2017. PMID: 28203148 Free PMC article.
-
Stable Gastric Pentadecapeptide BPC 157 May Recover Brain-Gut Axis and Gut-Brain Axis Function.Pharmaceuticals (Basel). 2023 Apr 30;16(5):676. doi: 10.3390/ph16050676. Pharmaceuticals (Basel). 2023. PMID: 37242459 Free PMC article. Review.
References
-
- Abdelmalik PA, Shannon P, Yiu A, Liang P, Adamchik Y, Weisspapir M, Samoilova M, Burnham WM, Carlen PL. Hypoglycemic seizures during transient hypoglycemia exacerbate hippocampal dysfunction. Neurobiol Dis. 2007;26:646–660. - PubMed
-
- Acosta-Martínez M, Levine JE. Regulation of KATP channel subunit gene expression by hyperglycemia in the mediobasal hypothalamus of female rats. Am J Physiol Endocrinol Metab. 2007;292:E1801–E1807. - PubMed
-
- Akasu T, Tsurusaki M, Shoji S. Depletion of glucose causes presynaptic inhibition of neuronal transmission in the rat dorsolateral septal nucleus. Synapse. 1996;24:125–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
