Requirement of myeloid cells for axon regeneration
- PMID: 18799670
- PMCID: PMC6671109
- DOI: 10.1523/JNEUROSCI.1447-08.2008
Requirement of myeloid cells for axon regeneration
Abstract
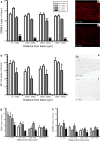
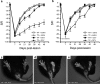
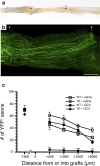
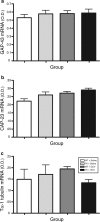
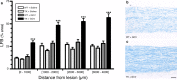
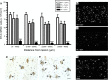
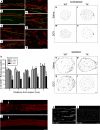
The role of CD11b+ myeloid cells in axonal regeneration was assessed using axonal injury models and CD11b-TK(mt-30) mice expressing a mutated HSV-1 thymidine kinase (TK) gene regulated by the myeloid-specific CD11b promoter. Continuous delivery of ganciclovir at a sciatic nerve lesion site greatly decreased the number of granulocytes/inflammatory monocytes and macrophages in the distal stump of CD11b-TK(mt-30) mice. Axonal regeneration and locomotor function recovery were severely compromised in ganciclovir-treated CD11b-TK(mt-30) mice. This was caused by an unsuitable growth environment rather than an altered regeneration capacity of neurons. In absence of CD11b+ cells, the clearance of inhibitory myelin debris was prevented, neurotrophin synthesis was abolished, and blood vessel formation/maintenance was severely compromised in the sciatic nerve distal stump. Spinal cord-injured axons also failed to regenerate through peripheral nerve grafts in the absence of CD11b+ cells. Therefore, myeloid cells support axonal regeneration and functional recovery by creating a growth-permissive milieu for injured axons.
Figures


References
-
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. - PubMed
-
- Bandtlow CE, Meyer M, Lindholm D, Spranger M, Heumann R, Thoenen H. Regional and cellular codistribution of interleukin 1 beta and nerve growth factor mRNA in the adult rat brain: possible relationship to the regulation of nerve growth factor synthesis. J Cell Biol. 1990;111:1701–1711. - PMC - PubMed
-
- Barrette B, Vallieres N, Dube M, Lacroix S. Expression profile of receptors for myelin-associated inhibitors of axonal regeneration in the intact and injured mouse central nervous system. Mol Cell Neurosci. 2007;34:519–538. - PubMed
-
- Brück W, Huitinga I, Dijkstra CD. Liposome-mediated monocyte depletion during wallerian degeneration defines the role of hematogenous phagocytes in myelin removal. J Neurosci Res. 1996;46:477–484. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
