Time constants of h current in layer ii stellate cells differ along the dorsal to ventral axis of medial entorhinal cortex
- PMID: 18799674
- PMCID: PMC2990529
- DOI: 10.1523/JNEUROSCI.3196-08.2008
Time constants of h current in layer ii stellate cells differ along the dorsal to ventral axis of medial entorhinal cortex
Abstract
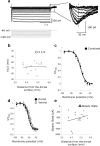
Chronic recordings in the medial entorhinal cortex of behaving rats have found grid cells, neurons that fire when the rat is in a hexagonal array of locations. Grid cells recorded at different dorsal-ventral anatomical positions show systematic changes in size and spacing of firing fields. To test possible mechanisms underlying these differences, we analyzed properties of the hyperpolarization-activated cation current I(h) in voltage-clamp recordings from stellate cells in entorhinal slices from different dorsal-ventral locations. The time constant of h current was significantly different between dorsal and ventral neurons. The time constant of h current correlated with membrane potential oscillation frequency and the time constant of the sag potential in the same neurons. Differences in h current could underlie differences in membrane potential oscillation properties and contribute to grid cell periodicity along the dorsal-ventral axis of medial entorhinal cortex.
Figures






References
-
- Acker CD, Kopell N, White JA. Synchronization of strongly coupled excitatory neurons: relating network behavior to biophysics. J Comput Neurosci. 2003;15:71–90. - PubMed
-
- Alonso A, Klink R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J Neurophysiol. 1993;70:128–143. - PubMed
-
- Alonso A, Llinás RR. Subthreshold Na-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature. 1989;342:175–177. - PubMed
-
- Barry C, Hayman R, Burgess N, Jeffery KJ. Experience-dependent rescaling of entorhinal grids. Nat Neurosci. 2007;10:682–684. - PubMed
-
- Brownlee H, Gao PP, Frisen J, Dreyfus C, Zhou R, Black IB. Multiple ephrins regulate hippocampal neurite outgrowth. J Comp Neurol. 2000;425:315–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
