Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice
- PMID: 18799677
- PMCID: PMC6671112
- DOI: 10.1523/JNEUROSCI.2674-08.2008
Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice
Abstract
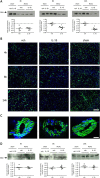
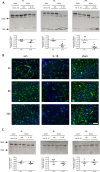
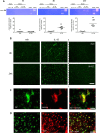
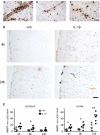
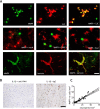
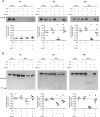
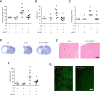
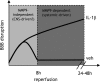
Systemic inflammatory events, such as infection, increase the risk of stroke and are associated with worse outcome, but the mediators of this clinically important effect are unknown. Our aim here was to elucidate mechanisms contributing to the detrimental effects of systemic inflammation on mild ischemic brain injury in mice. Systemic inflammation was induced in mice by peripheral interleukin-1beta (IL-1beta) challenge and focal cerebral ischemia by transient middle cerebral artery occlusion (MCAo). Systemic inflammation caused an alteration in the kinetics of blood-brain barrier (BBB) disruption through conversion of a transient to a sustained disruption of the tight junction protein, claudin-5, and also markedly exacerbated disruption to the cerebrovascular basal lamina protein, collagen-IV. These alterations were associated with a systemic inflammation-induced increase in neurovascular gelatinolytic activity that was mediated by a fivefold increase in neutrophil-derived matrix metalloproteinase-9 (MMP-9) in the brains of IL-1beta-challenged mice after MCAo. Specific inhibition of MMP-9 abrogated the effects of systemic inflammation on the sustained but not the acute disruption of claudin-5, which was associated with phosphorylation of cerebrovascular myosin light chain. MMP-9 inhibition also attenuated the deleterious impact of systemic inflammation on brain damage, edema, neurological deficit, and incidence of hemorrhagic transformation. These data indicate that a transformation from transient to sustained BBB disruption caused by enhanced neutrophil-derived neurovascular MMP-9 activity is a critical mechanism underlying the exacerbation of ischemic brain injury by systemic inflammation. These mechanisms may contribute to the poor clinical outcome in stroke patients presenting with antecedent infection.
Figures
References
-
- Afonso PV, Ozden S, Prevost MC, Schmitt C, Seilhean D, Weksler B, Couraud PO, Gessain A, Romero IA, Ceccaldi PE. Human blood-brain barrier disruption by retroviral-infected lymphocytes: role of myosin light chain kinase in endothelial tight-junction disorganization. J Immunol. 2007;179:2576–2583. - PubMed
-
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. - PubMed
-
- Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
