Decreased striatal dopamine release underlies increased expression of long-term synaptic potentiation at corticostriatal synapses 24 h after 3-nitropropionic-acid-induced chemical hypoxia
- PMID: 18799690
- PMCID: PMC2724653
- DOI: 10.1523/JNEUROSCI.5698-07.2008
Decreased striatal dopamine release underlies increased expression of long-term synaptic potentiation at corticostriatal synapses 24 h after 3-nitropropionic-acid-induced chemical hypoxia
Abstract
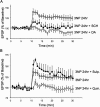
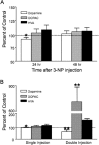
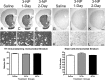
The striatum is particularly sensitive to the irreversible inhibitor of succinate dehydrogenase 3-nitropropionic acid (3-NP). In the present study, we examined early changes in behavior and dopamine and glutamate synaptic physiology created by a single systemic injection of 3-NP in Fischer 344 rats. Hindlimb dystonia was seen 2 h after 3-NP injections, and rats performed poorly on balance beam and rotarod motor tests 24 h later. Systemic 3-NP increased NMDA receptor-dependent long-term potentiation (LTP) at corticostriatal synapses over the same time period. The 3-NP-induced corticostriatal LTP was not attributable to increased NMDA receptor number or function, because 3-NP did not change MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine] binding or NMDA/AMPA receptor current ratios. The LTP seen 24 h after 3-NP was D(1) receptor dependent and reversed by exogenous addition of dopamine or a D(2) receptor agonist to brain slices. HPLC and fast-scan cyclic voltammetry revealed a decrease in dopamine content and release in rats injected 24 h earlier with 3-NP, and much like the enhanced LTP, dopamine changes were reversed by 48 h. Tyrosine hydroxylase expression was not changed, and there was no evidence of striatal cell loss at 24-48 h after 3-NP exposure. Sprague Dawley rats showed similar physiological responses to systemic 3-NP, albeit with reduced sensitivity. Thus, 3-NP causes significant changes in motor behavior marked by parallel changes in striatal dopamine release and corticostriatal synaptic plasticity.
Figures





References
-
- Akopian G, Walsh JP. Reduced expression of short- and long-term facilitation at aged corticostriatal synapses. Synapse. 2006;60:223–238. - PubMed
-
- Akopian G, Musleh W, Smith R, Walsh JP. Functional state of presynaptic terminals influences the expression of short- and long-term plasticity at corticostriatal synapses. Synapse. 2000;38:271–280. - PubMed
-
- Aspey BS, Cohen S, Patel Y, Terruli M, Harrison MJ. Middle cerebral artery occlusion in the rat: consistent protocol for a model of stroke. Neuropathol Appl Neurobiol. 1998;24:487–497. - PubMed
-
- Aspey BS, Taylor FL, Terruli M, Harrison MJ. Temporary middle cerebral artery occlusion in the rat: consistent protocol for a model of stroke and reperfusion. Neuropathol Appl Neurobiol. 2000;26:232–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
