Highly sensitive amperometric immunosensor for detection of Plasmodium falciparum histidine-rich protein 2 in serum of humans with malaria: comparison with a commercial kit
- PMID: 18799699
- PMCID: PMC2576608
- DOI: 10.1128/JCM.01022-08
Highly sensitive amperometric immunosensor for detection of Plasmodium falciparum histidine-rich protein 2 in serum of humans with malaria: comparison with a commercial kit
Abstract
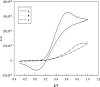
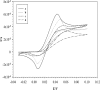
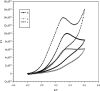
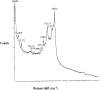
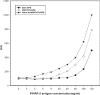
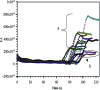
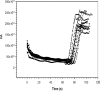
A disposable amperometric immunosensor was developed for the detection of Plasmodium falciparum histidine-rich protein 2 (PfHRP-2) in the sera of humans with P. falciparum malaria. For this purpose, disposable screen-printed electrodes (SPEs) were modified with multiwall carbon nanotubes (MWCNTs) and Au nanoparticles. The electrodes were characterized by cyclic voltammetry, scanning electron microscopy, and Raman spectroscopy. In order to study the immunosensing performances of modified electrodes, a rabbit anti-PfHRP-2 antibody (as the capturing antibody) was first immobilized on an electrode. Further, the electrode was exposed to a mouse anti-PfHRP-2 antibody from a serum sample (as the revealing antibody), followed by a rabbit anti-mouse immunoglobulin G-alkaline phosphatase conjugate. The immunosensing experiments were performed on bare SPEs, MWCNT-modified SPEs, and Au nanoparticle- and MWCNT-modified SPEs (Nano-Au/MWCNT/SPEs) for the amperometric detection of PfHRP-2 in a solution of 0.1 M diethanolamine buffer, pH 9.8, by applying a potential of 450 mV at the working electrode. Nano-Au/MWCNT/SPEs yielded the highest-level immunosensing performance among the electrodes, with a detection limit of 8 ng/ml. The analytical results of immunosensing experiments with human serum samples were compared with the results of a commercial Paracheck Pf test, as well as the results of microscopy. The specificities, sensitivities, and positive and negative predictive values of the Paracheck Pf and amperometric immunosensors were calculated by taking the microscopy results as the "gold standard." The Paracheck Pf kit exhibited a sensitivity of 79% (detecting 34 of 43 positive samples; 95% confidence interval [CI], 75 to 86%) and a specificity of 81% (correctly identifying 57 of 70 negative samples; 95% CI, 76 to 92%), whereas the developed amperometric immunosensor showed a sensitivity of 96% (detecting 41 of 43 positive samples; 95% CI, 93 to 98%) and a specificity of 94% (correctly identifying 66 of 70 negative samples; 95% CI, 92 to 99%). The developed method is more sensitive and specific than the Paracheck Pf kit.
Figures

References
-
- Abad, J. M., S. F. L. Mertens, M. Pita, V. M. Fernandez, and D. J. Schiffrin. 2005. Functionalization of thioctic acid-capped gold nanoparticles for specific immobilization of histidine-tagged proteins. J. Am. Chem. Soc. 1275689-5694. - PubMed
-
- Bharathi, S., and M. Nogami. 2001. A glucose biosensor based on electrodeposited bio-composites of gold nanoparticles and glucose oxidase enzyme. Analyst 1261919-1922. - PubMed
-
- Broek, I. V. D., O. Hill, F. Gordillo, B. Angarita, P. Hamade, H. Counihan, and J. Guthmann. 2006. Evaluation of three rapid tests for diagnosis of P. falciparum and P. vivax malaria in Colombia. Am. J. Trop. Med. Hyg. 751209-1215. - PubMed
-
- Brown, K. R., A. P. Fox, and M. J. Natan. 1996. Morphology-dependent electrochemistry of cytochrome c at Au colloid-modified SnO2 electrodes. J. Am. Chem. Soc. 1181154-1157.
-
- Cai, H., C. Xu, P. G. He, and Y. Z. Fang. 2001. Colloid Au-enhanced DNA immobilization for the electrochemical detection of sequence-specific DNA. J. Electroanal. Chem. 51078-85.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

