The molecular logic of Notch signaling--a structural and biochemical perspective
- PMID: 18799787
- PMCID: PMC2696053
- DOI: 10.1242/jcs.035683
The molecular logic of Notch signaling--a structural and biochemical perspective
Abstract
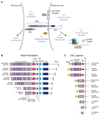
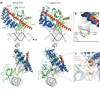
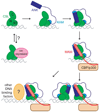
The Notch signaling pathway constitutes an ancient and conserved mechanism for cell-cell communication in metazoan organisms, and has a central role both in development and in adult tissue homeostasis. Here, we summarize structural and biochemical advances that contribute new insights into three central facets of canonical Notch signal transduction: (1) ligand recognition, (2) autoinhibition and the switch from protease resistance to protease sensitivity, and (3) the mechanism of nuclear-complex assembly and the induction of target-gene transcription. These advances set the stage for future mechanistic studies investigating ligand-dependent activation of Notch receptors, and serve as a foundation for the development of mechanism-based inhibitors of signaling in the treatment of cancer and other diseases.
Figures

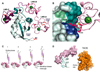

References
-
- Aster JC, Robertson ES, Hasserjian RP, Turner JR, Kieff E, Sklar J. Oncogenic forms of NOTCH1 lacking either the primary binding site for RBP-Jkappa or nuclear localization sequences retain the ability to associate with RBP-Jkappa and activate transcription. J Biol Chem. 1997;272:11336–11343. - PubMed
-
- Bailey AM, Posakony JW. Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev. 1995;9:2609–2622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

