Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization
- PMID: 18800071
- PMCID: PMC5527972
- DOI: 10.1038/npp.2008.144
Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization
Abstract
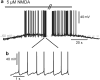
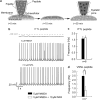
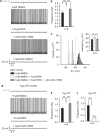
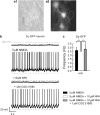
Bursting activity of striatal medium spiny neurons results from membrane potential oscillations between a down- and an upstate that could be regulated by G-protein-coupled receptors. Among these, dopamine D(2) and adenosine A(2A) receptors are highly enriched in striatal neurons and exhibit strong interactions whose physiological significance and molecular mechanisms remain partially unclear. More particularly, respective involvements of common intracellular signaling cascades and A(2A)-D(2) receptor heteromerization remain unknown. Here we show, by performing perforated-patch-clamp recordings on brain slices and loading competitive peptides, that D(2) and A(2A) receptors regulate the induction by N-methyl-D-aspartate of a depolarized membrane potential plateau through mechanisms relying upon specific protein-protein interactions. Indeed, D(2) receptor activation abolished transitions between a hyperpolarized resting potential and a depolarized plateau potential by regulating the Ca(V)1.3a calcium channel activity through interactions with scaffold proteins Shank1/3. Noticeably, A(2A) receptor activation had no effect per se but fully reversed the effects of D(2) receptor activation through a mechanism in which A(2A)-D(2) receptors heteromerization is strictly mandatory, demonstrating therefore a first direct physiological relevance of these heteromers. Our results show that membrane potential transitions and firing patterns in striatal neurons are tightly controlled by D(2) and A(2A) receptors through specific protein-protein interactions including A(2A)-D(2) receptors heteromerization.
Conflict of interest statement
The authors declare that except for income received from the primary employer no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Figures
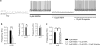


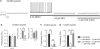
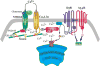
References
-
- Agnati LF, Ferré S, Lluis C, Franco R, Fuxe K. Molecular mechanisms and therapeutic implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol Rev. 2003;55:509–550. - PubMed
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, et al. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–46749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

