Natural products as inspiration for the development of asymmetric catalysis
- PMID: 18800131
- PMCID: PMC2562237
- DOI: 10.1038/nature07370
Natural products as inspiration for the development of asymmetric catalysis
Abstract
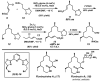
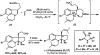
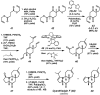
Biologically active natural products often contain particularly challenging structural features and functionalities in terms of synthesis. Perhaps the greatest difficulties are those caused by issues of stereochemistry. A useful strategy for synthesizing such molecules is to devise methods of bond formation that provide opportunities for using enantioselective catalysis. In using this tactic, the desire for a particular target structure ultimately drives the development of catalytic methods. New enantioselective catalytic methods contribute to a greater fundamental understanding of how bonds can be constructed and lead to valuable synthetic technologies that are useful for a variety of applications.
Figures

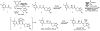




References
-
- Corey EJ, Cheng X-M. The Logic of Chemical Synthesis. Wiley; New York: 1995. pp. 1–91.
-
- Jacobsen EN, Pfaltz A, Yamamoto H, editors. Comprehensive Asymmetric Catalysis, Vol. I–III. Springer-Verlag; Berlin: 2000.
-
- Jacobsen EN, Pfaltz A, Yamamoto H, editors. Comprehensive Asymmetric Catalysis, Supplement 1 & 2. Springer-Verlag; Berlin: 2004.
-
- Hoveyda AH. Asymmetric Catalysis in Target-Oriented Synthesis. In: Vögtle F, Stoddart JF, Shibasaki M, editors. Stimulating Concepts in Chemistry. Wiley-VCH; Weinheim: 2000. pp. 145–160.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

