Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow
- PMID: 18800171
- PMCID: PMC2535565
- DOI: 10.1371/journal.pone.0003234
Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow
Abstract
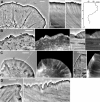
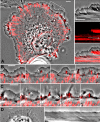
Dynamic actin network at the leading edge of the cell is linked to the extracellular matrix through focal adhesions (FAs), and at the same time it undergoes retrograde flow with different dynamics in two distinct zones: the lamellipodium (peripheral zone of fast flow), and the lamellum (zone of slow flow located between the lamellipodium and the cell body). Cell migration involves expansion of both the lamellipodium and the lamellum, as well as formation of new FAs, but it is largely unknown how the position of the boundary between the two flow zones is defined, and how FAs and actin flow mutually influence each other. We investigated dynamic relationship between focal adhesions and the boundary between the two flow zones in spreading cells. Nascent FAs first appeared in the lamellipodium. Within seconds after the formation of new FAs, the rate of actin flow decreased locally, and the lamellipodium/lamellum boundary advanced towards the new FAs. Blocking fast actin flow with cytochalasin D resulted in rapid dissolution of nascent FAs. In the absence of FAs (spreading on poly-L-lysine-coated surfaces) retrograde flow was uniform and the velocity transition was not observed. We conclude that formation of FAs depends on actin dynamics, and in its turn, affects the dynamics of actin flow by triggering transition from fast to slow flow. Extension of the cell edge thus proceeds through a cycle of lamellipodium protrusion, formation of new FAs, advance of the lamellum, and protrusion of the lamellipodium from the new base.
Conflict of interest statement
Figures


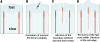
References
-
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. - PubMed
-
- Mogilner A, Oster G. Polymer motors: pushing out the front and pulling up the back. Curr Biol. 2003;13:R721–733. - PubMed
-
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. - PubMed
-
- Kaverina I, Krylyshkina O, Small JV. Regulation of substrate adhesion dynamics during cell motility. Int J Biochem Cell Biol. 2002;34:746–761. - PubMed
-
- Webb DJ, Brown CM, Horwitz AF. Illuminating adhesion complexes in migrating cells: moving toward a bright future. Curr Opin Cell Biol. 2003;15:614–620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

