Synthesis, ex vivo evaluation, and radiolabeling of potent 1,5-diphenylpyrrolidin-2-one cannabinoid subtype-1 receptor ligands as candidates for in vivo imaging
- PMID: 18800770
- PMCID: PMC2587418
- DOI: 10.1021/jm800416m
Synthesis, ex vivo evaluation, and radiolabeling of potent 1,5-diphenylpyrrolidin-2-one cannabinoid subtype-1 receptor ligands as candidates for in vivo imaging
Abstract
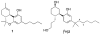
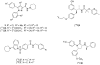
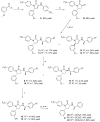
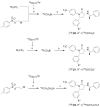
We have reported that [methyl- (11)C] (3 R,5 R)-5-(3-methoxyphenyl)-3-[(R)-1-phenylethylamino]-1-(4-trifluoromethylphenyl)pyrrolidin-2-one ([(11)C] 8, [(11)C]MePPEP) binds with high selectivity to cannabinoid type-1 (CB 1) receptors in monkey brain in vivo. We now describe the synthesis of 8 and four analogues, namely, the 4-fluorophenyl (16, FMePPEP), 3-fluoromethoxy (20, FMPEP), 3-fluoromethoxy- d 2 (21, FMPEP- d 2), and 3-fluoroethoxy analogues (22, FEPEP), and report their activity in an ex vivo model designed to identify compounds suitable for use as positron emission tomography (PET) ligands. These ligands exhibited high, selective potency at CB 1 receptors in vitro (K b < 1 nM). Each ligand (30 microg/kg, iv) was injected into rats under baseline and pretreatment conditions (3, rimonabant, 10 mg/kg, iv) and quantified at later times in frontal cortex ex vivo with liquid chromatography-mass spectrometry (LC-MS) detection. Maximal ligand uptakes were high (22.6-48.0 ng/g). Under pretreatment, maximal brain uptakes were greatly reduced (6.5-17.3 ng/g). Since each ligand readily entered brain and bound with high selectivity to CB 1 receptors, we then established and here describe methods for producing [(11)C] 8, [(11)C] 16, and [(18)F] 20- 22 in adequate activities for evaluation as candidate PET radioligands in vivo.
Figures


References
-
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu. Rev. Pharmacol. Toxicol. 2005;46:101–122. - PubMed
-
- Lambert DM, Fowler CJ. The endocannabinoid system: drug targets, lead compounds, and potential therapeutic applications. J. Med. Chem. 2005;48:5059–5087. - PubMed
-
- Mechoulam R, Gaoni Y. Absolute configuration of Δ1-tetrahydrocannabinol major active constituent of hashish. Tetrahedron Lett. 1967:1109–1111. - PubMed
-
- Johnson MR, Melvin LS. Cannabinoids as Therapeutic Agents. CRC Press; Boca Raton, FL: 1986. pp. 121–145.
-
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

