Altered homeostasis of CD4(+) FoxP3(+) regulatory T-cell subpopulations in systemic lupus erythematosus
- PMID: 18800986
- PMCID: PMC2691785
- DOI: 10.1111/j.1365-2567.2008.02937.x
Altered homeostasis of CD4(+) FoxP3(+) regulatory T-cell subpopulations in systemic lupus erythematosus
Abstract
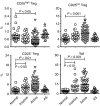
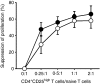
The role of naturally occurring regulatory T cells (Treg), known to be phenotypically heterogeneous, in controlling the expression of systemic lupus erythematosus (SLE) is incompletely defined. Therefore, different subpopulations of CD4(+) FoxP3(+) Tregs in patients with active or inactive SLE were investigated and compared with those of healthy subjects and patients with ankylosing spondylitis (AS). Characterization of different subsets of circulating CD4(+) FoxP3(+) Tregs was examined using flow cytometry. CD4(+) CD25(high) T cells were sorted and examined for suppressive activity in vitro. The results showed first that a significant decrease in the frequency of CD4(+) CD25(high) FoxP3(+) T cells was present in patients with active SLE (n = 58), compared with healthy controls (n = 36) and AS patients (n = 23). In contrast, the frequencies of CD25(low) FoxP3(+) and CD25(-) FoxP3(+) CD4(+) T cells were significantly increased in patients with active SLE by comparison with the control subjects. The elevation of these two putative Treg subpopulations was associated with lower plasma levels of complement C3 and C4 in patients with SLE. In addition, the ratios of the three subsets of CD4(+) FoxP3(+) Tregs versus effector T cells (CD4(+) CD25(+) FoxP3(-)) were inversely correlated with the titer of anti-double-stranded DNA IgG in patients with inactive, but not active, SLE. These results suggest that the pathogenesis of SLE may be associated with a defect in the homeostatic control of different Treg subsets.
Figures

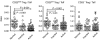
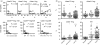
References
-
- Tan EM. Autoantibodies and autoimmunity: a three-decade perspective. A tribute to Henry G. Kunkel. Ann N Y Acad Sci. 1997;815:1–14. - PubMed
-
- Crispin JC, Martinez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2003;21:273–6. - PubMed
-
- Liu MF, Wang CR, Fung LL, Wu CR. Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol. 2004;59:198–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

