The TRAPP complex: insights into its architecture and function
- PMID: 18801063
- PMCID: PMC3417770
- DOI: 10.1111/j.1600-0854.2008.00833.x
The TRAPP complex: insights into its architecture and function
Abstract
Vesicle-mediated transport is a process carried out by virtually every cell and is required for the proper targeting and secretion of proteins. As such, there are numerous players involved to ensure that the proteins are properly localized. Overall, transport requires vesicle budding, recognition of the vesicle by the target membrane and fusion of the vesicle with the target membrane resulting in delivery of its contents. The initial interaction between the vesicle and the target membrane has been referred to as tethering. Because this is the first contact between the two membranes, tethering is critical to ensuring that specificity is achieved. It is therefore not surprising that there are numerous 'tethering factors' involved ranging from multisubunit complexes, coiled-coil proteins and Rab guanosine triphosphatases. Of the multisubunit tethering complexes, one of the best studied at the molecular level is the evolutionarily conserved TRAPP complex. There are two forms of this complex: TRAPP I and TRAPP II. In yeast, these complexes function in a number of processes including endoplasmic reticulum-to-Golgi transport (TRAPP I) and an ill-defined step at the trans Golgi (TRAPP II). Because the complex was first reported in 1998 (1), there has been a decade of studies that have clarified some aspects of its function but have also raised further questions. In this review, we will discuss recent advances in our understanding of yeast and mammalian TRAPP at the structural and functional levels and its role in disease while trying to resolve some apparent discrepancies and highlighting areas for future study.
Figures

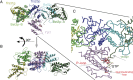
References
-
- Kim MS, Yi MJ, Lee KH, Wagner J, Munger C, Kim YG, Whiteway M, Cygler M, Oh BH, Sacher M. Biochemical and crystallographic studies reveal a specific interaction between TRAPP subunits Trs33p and Bet3p. Traffic 2005;6:1183–1195. - PubMed
-
- Dong G, Hutagalung AH, Fu C, Novick P, Reinisch KM. The structures of exocyst subunit Exo70p and the Exo84p C‐terminal domains reveal a common motif. Nat Struct Mol Biol 2005;12:1094–1100. - PubMed
-
- Sivaram MV, Furgason ML, Brewer DN, Munson M. The structure of the exocyst subunit Sec6p defines a conserved architecture with diverse roles. Nat Struct Mol Biol 2006;13:555–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

