Inversion of allosteric effect of arginine on N-acetylglutamate synthase, a molecular marker for evolution of tetrapods
- PMID: 18801197
- PMCID: PMC2566978
- DOI: 10.1186/1471-2091-9-24
Inversion of allosteric effect of arginine on N-acetylglutamate synthase, a molecular marker for evolution of tetrapods
Abstract
Background: The efficient conversion of ammonia, a potent neurotoxin, into non-toxic metabolites was an essential adaptation that allowed animals to move from the aquatic to terrestrial biosphere. The urea cycle converts ammonia into urea in mammals, amphibians, turtles, snails, worms and many aquatic animals and requires N-acetylglutamate (NAG), an essential allosteric activator of carbamylphosphate synthetase I (CPSI) in mammals and amphibians, and carbamylphosphate synthetase III (CPSIII) in fish and invertebrates. NAG-dependent CPSI and CPSIII catalyze the formation of carbamylphosphate in the first and rate limiting step of ureagenesis. NAG is produced enzymatically by N-acetylglutamate synthase (NAGS), which is also found in bacteria and plants as the first enzyme of arginine biosynthesis. Arginine is an allosteric inhibitor of microbial and plant NAGS, and allosteric activator of mammalian NAGS.
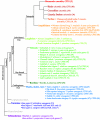
Results: Information from mutagenesis studies of E. coli and P. aeruginosa NAGS was combined with structural information from the related bacterial N-acetylglutamate kinases to identify four residues in mammalian NAGS that interact with arginine. Substitutions of these four residues were engineered in mouse NAGS and into the vertebrate-like N-acetylglutamate synthase-kinase (NAGS-K) of Xanthomonas campestris, which is inhibited by arginine. All mutations resulted in arginine losing the ability to activate mouse NAGS, and inhibit X. campestris NAGS-K. To examine at what point in evolution inversion of arginine effect on NAGS occur, we cloned NAGS from fish and frogs and examined the arginine response of their corresponding proteins. Fish NAGS were partially inhibited by arginine and frog NAGS were activated by arginine.
Conclusion: Difference in arginine effect on bacterial and mammalian NAGS most likely stems from the difference in the type of conformational change triggered by arginine binding to these proteins. The change from arginine inhibition of NAGS to activation was gradual, from complete inhibition of bacterial NAGS, to partial inhibition of fish NAGS, to activation of frog and mammalian NAGS. This change also coincided with the conquest of land by amphibians and mammals.
Figures

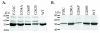

References
-
- Cohen SS. On biochemical variability and innovation. Science. 1963;139:1017–1026. - PubMed
-
- Mommsen TP, Walsh PJ. Evolution of urea synthesis in vertebrates: the piscine connection. Science. 1989;243:72–75. - PubMed
-
- Brusilow SW, Horwich AL. Urea Cycle Enzymes. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editor. The Metabolic & Molecular Bases of Inherited Disease. Vol. 2. McGraw-Hill; 2001. pp. 1909–1963.
-
- Ip YK, Chew SF, Randall DJ. Five tropical air-breathing fishes, six different strategies to defend against ammonia toxicity on land. Physiol Biochem Zool. 2004;77:768–782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

