Temperature-dependent production of pseudoinfectious dengue reporter virus particles by complementation
- PMID: 18801552
- PMCID: PMC3428711
- DOI: 10.1016/j.virol.2008.08.021
Temperature-dependent production of pseudoinfectious dengue reporter virus particles by complementation
Abstract
Dengue virus (DENV) is a mosquito-borne flavivirus responsible for 50 to 100 million human infections each year, highlighting the need for a safe and effective vaccine. In this study, we describe the production of pseudoinfectious DENV reporter virus particles (RVPs) using two different genetic complementation approaches, including the creation of cell lines that release reporter viruses in an inducible fashion. In contrast to studies with West Nile virus (WNV), production of infectious DENV RVPs was temperature-dependent; the yield of infectious DENV RVPs at 37 degrees C is significantly reduced in comparison to experiments conducted at lower temperatures or with WNV. This reflects both a significant reduction in the rate of infectious DENV RVP release over time, and the more rapid decay of infectious DENV RVPs at 37 degrees C. Optimized production approaches allow the production of DENV RVPs with titers suitable for the study of DENV entry, assembly, and the analysis of the humoral immune response of infected and vaccinated individuals.
Figures
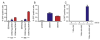

References
-
- Alvarez M, Rodriguez-Roche R, Bernardo L, Vazquez S, Morier L, Gonzalez D, Castro O, Kouri G, Halstead SB, Guzman MG. Dengue hemorrhagic fever caused by sequential dengue 1–3 virus infections over a long time interval: Havana epidemic, 2001–2002. Am. J. Trop. Med. Hyg. 2006;75(6):1113–1117. - PubMed
-
- Andrewes C.H.a.E., W.J. Observations on anti-phage sera. I. ‘The percentage law’. Br. J. Exp. Pathol. 1933;14:367–376.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

