Molecular recognition of corticotropin-releasing factor by its G-protein-coupled receptor CRFR1
- PMID: 18801728
- PMCID: PMC2583312
- DOI: 10.1074/jbc.M805749200
Molecular recognition of corticotropin-releasing factor by its G-protein-coupled receptor CRFR1
Abstract
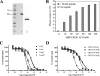
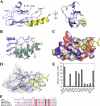
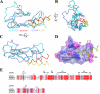
The bimolecular interaction between corticotropin-releasing factor (CRF), a neuropeptide, and its type 1 receptor (CRFR1), a class B G-protein-coupled receptor (GPCR), is crucial for activation of the hypothalamic-pituitary-adrenal axis in response to stress, and has been a target of intense drug design for the treatment of anxiety, depression, and related disorders. As a class B GPCR, CRFR1 contains an N-terminal extracellular domain (ECD) that provides the primary ligand binding determinants. Here we present three crystal structures of the human CRFR1 ECD, one in a ligand-free form and two in distinct CRF-bound states. The CRFR1 ECD adopts the alpha-beta-betaalpha fold observed for other class B GPCR ECDs, but the N-terminal alpha-helix is significantly shorter and does not contact CRF. CRF adopts a continuous alpha-helix that docks in a hydrophobic surface of the ECD that is distinct from the peptide-binding site of other class B GPCRs, thereby providing a basis for the specificity of ligand recognition between CRFR1 and other class B GPCRs. The binding of CRF is accompanied by clamp-like conformational changes of two loops of the receptor that anchor the CRF C terminus, including the C-terminal amide group. These structural studies provide a molecular framework for understanding peptide binding and specificity by the CRF receptors as well as a template for designing potent and selective CRFR1 antagonists for therapeutic applications.
Figures
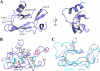



References
-
- Vale, W., Spiess, J., Rivier, C., and Rivier, J. (1981) Science 213 1394-1397 - PubMed
-
- Hsu, S. Y., and Hsueh, A. J. (2001) Nat. Med. 7 605-611 - PubMed
-
- Vaughan, J., Donaldson, C., Bittencourt, J., Perrin, M. H., Lewis, K., Sutton, S., Chan, R., Turnbull, A. V., Lovejoy, D., Rivier, C., Rivier, J., Sawchenko, P. E., and Vale, W. (1995) Nature 378 287-292 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

