TRPML3 mutations cause impaired mechano-electrical transduction and depolarization by an inward-rectifier cation current in auditory hair cells of varitint-waddler mice
- PMID: 18801844
- PMCID: PMC2655368
- DOI: 10.1113/jphysiol.2008.156992
TRPML3 mutations cause impaired mechano-electrical transduction and depolarization by an inward-rectifier cation current in auditory hair cells of varitint-waddler mice
Abstract
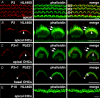
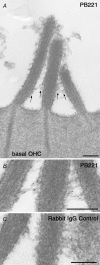
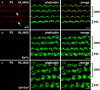
TRPML3 (mucolipin-3) belongs to one of the transient-receptor-potential (TRP) ion channel families. Mutations in the Trpml3 gene cause disorganization of the stereociliary hair bundle, structural aberrations in outer and inner hair cells and stria vascularis defects, leading to deafness in the varitint-waddler (Va) mouse. Here we refined the stereociliary localization of TRPML3 and investigated cochlear hair cell function in varitint-waddler (Va(J)) mice carrying the TRPML3<I362T/A419P> mutations. Using a TRPML3-specific antibody we detected a approximately 68 kDa protein with near-equal expression levels in cochlea and vestibule of wild-type and Va(J) mutants. At postnatal days 3 and 5, we observed abundant localization of TRPML3 at the base of stereocilia near the position of the ankle links. This stereociliary localization domain was absent in Va(J) heterozygotes and homozygotes. Electrophysiological recordings revealed reduced mechano-electrical transducer currents in hair cells from Va(J)/+ and Va(J)/Va(J) mice. Furthermore, FM1-43 uptake and [(3)H]gentamicin accumulation were decreased in hair cells in cultured organs of Corti from Va(J)/+ and Va(J)/Va(J) mice. We propose that TRPML3 plays a critical role at the ankle-link region during hair-bundle growth and that an adverse effect of mutant TRPML3 on bundle development and mechano-electrical transduction is the main cause of hearing loss in Va(J)/+ mutant mice. Outer hair cells of Va(J)/Va(J) mice additionally had depolarized resting potentials due to an inwardly rectifying leak conductance formed by the mutant channels, leading over time to hair-cell degeneration and contributing to their deafness. Our findings argue against TRPML3 being a component of the hair-cell transducer channel.
Figures



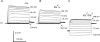


Similar articles
-
The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration.Proc Natl Acad Sci U S A. 2008 Jan 8;105(1):353-8. doi: 10.1073/pnas.0707963105. Epub 2007 Dec 27. Proc Natl Acad Sci U S A. 2008. PMID: 18162548 Free PMC article.
-
TRPML3 and hearing loss in the varitint-waddler mouse.Biochim Biophys Acta. 2007 Aug;1772(8):1028-31. doi: 10.1016/j.bbadis.2007.01.007. Epub 2007 Jan 23. Biochim Biophys Acta. 2007. PMID: 17329082 Review.
-
The varitint-waddler mouse phenotypes and the TRPML3 ion channel mutation: cause and consequence.Pflugers Arch. 2008 Nov;457(2):463-73. doi: 10.1007/s00424-008-0523-4. Epub 2008 May 27. Pflugers Arch. 2008. PMID: 18504603 Review.
-
Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice.Proc Natl Acad Sci U S A. 2002 Nov 12;99(23):14994-9. doi: 10.1073/pnas.222425399. Epub 2002 Oct 25. Proc Natl Acad Sci U S A. 2002. PMID: 12403827 Free PMC article.
-
A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse.Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19583-8. doi: 10.1073/pnas.0709846104. Epub 2007 Nov 28. Proc Natl Acad Sci U S A. 2007. PMID: 18048323 Free PMC article.
Cited by
-
The how and why of identifying the hair cell mechano-electrical transduction channel.Pflugers Arch. 2015 Jan;467(1):73-84. doi: 10.1007/s00424-014-1606-z. Epub 2014 Sep 23. Pflugers Arch. 2015. PMID: 25241775 Free PMC article. Review.
-
Genetic inactivation of Trpml3 does not lead to hearing and vestibular impairment in mice.PLoS One. 2010 Dec 13;5(12):e14317. doi: 10.1371/journal.pone.0014317. PLoS One. 2010. PMID: 21179200 Free PMC article.
-
TRPMLs: in sickness and in health.Am J Physiol Renal Physiol. 2009 Jun;296(6):F1245-54. doi: 10.1152/ajprenal.90522.2008. Epub 2009 Jan 21. Am J Physiol Renal Physiol. 2009. PMID: 19158345 Free PMC article. Review.
-
Life and death of sensory hair cells expressing constitutively active TRPML3.J Biol Chem. 2009 May 15;284(20):13823-13831. doi: 10.1074/jbc.M809045200. Epub 2009 Mar 19. J Biol Chem. 2009. PMID: 19299509 Free PMC article.
-
Constitutive activity of the human TRPML2 channel induces cell degeneration.J Biol Chem. 2010 Jan 22;285(4):2771-82. doi: 10.1074/jbc.M109.046508. Epub 2009 Nov 23. J Biol Chem. 2010. PMID: 19940139 Free PMC article.
References
-
- Adato A, Lefevre G, Delprat B, Michel V, Michalski N, Chardenoux S, Weil D, El-Amraoui A, Petit C. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum Mol Genet. 2005;14:3921–3932. - PubMed
-
- Anniko M, Wroblewski R. Ionic environment of cochlear hair cells. Hear Res. 1986;22:279–293. - PubMed
-
- Atiba-Davies M, Noben-Trauth K. TRPML3 and hearing loss in the varitint-waddler mouse. Biochim Biophys Acta. 2007;1772:1028–1031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

