Functional characterization and structural modeling of obesity associated mutations in the melanocortin 4 receptor
- PMID: 18801902
- PMCID: PMC2732289
- DOI: 10.1210/en.2008-0721
Functional characterization and structural modeling of obesity associated mutations in the melanocortin 4 receptor
Abstract
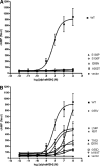
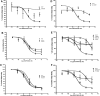
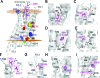
Mutations in the melanocortin 4 receptor (MC4R) gene are the most common known cause of monogenic human obesity. The MC4R gene was sequenced in 2000 subjects with severe early-onset obesity. We detected seven different nonsense and 19 nonsynonymous mutations in a total of 94 probands, some of which have been reported previously by others. We functionally characterized the 11 novel obesity associated missense mutations. Seven of these mutants (L54P, E61K, I69T, S136P, M161T, T162I, and I269N) showed impaired cell surface trafficking, reduced level of maximal binding of the radioligand [125I]NDP-MSH, and reduced ability to generate cAMP in response to ligand. Four mutant MC4Rs (G55V, G55D, S136F, and A303T) displayed cell surface expression and agonist binding similar to the wild-type receptor but showed impaired cAMP production, suggesting that these residues are likely to be critical for conformational rearrangement essential for receptor activation. Homology modeling of these mutants using a model of MC4R based on the crystal structure of the beta2-adrenoreceptor was used to provide insights into the possible structural basis for receptor dysfunction. Transmembrane (TM) domains 1, 3, 6, 7, and peripheral helix 8 appear to participate in the agonist-induced conformational rearrangement necessary for coupling of ligand binding to signaling. We conclude that G55V, G55D, S136F, and A303T mutations are likely to strengthen helix-helix interactions between TM1 and TM2, TM3 and TM6, and TM7 and helix 8, respectively, preventing relative movement of these helices during receptor activation. The combination of functional studies and structural modeling of naturally occurring pathogenic mutations in MC4R can provide valuable information regarding the molecular mechanism of MC4R activation and its dysfunction in human disease.
Figures


References
-
- Cone RD 2005 Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578 - PubMed
-
- Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S 2003 Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 348:1085–1095 - PubMed
-
- Alharbi KK, Spanakis E, Tan K, Smith MJ, Aldahmesh MA, O'Dell SD, Sayer AA, Lawlor DA, Ebrahim S, Davey Smith G, O'Rahilly S, Farooqi S, Cooper C, Phillips DI, Day IN 2007 Prevalence and functionality of paucimorphic and private MC4R mutations in a large, unselected European British population, scanned by meltMADGE. Hum Mutat 28:294–302 - PubMed
-
- Lubrano-Berthelier C, Dubern B, Lacorte JM, Picard F, Shapiro A, Zhang S, Bertrais S, Hercberg S, Basdevant A, Clement K, Vaisse C 2006 Melanocortin 4 receptor mutations in a large cohort of severely obese adults: prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. J Clin Endocrinol Metab 91:1811–1818 - PubMed
-
- Stutzmann F, Tan K, Vatin V, Dina C, Jouret B, Tichet J, Balkau B, Potoczna N, Horber F, O'Rahilly S, Farooqi IS, Froguel P, Meyre D 2008 Prevalence of melanocortin-4 receptor deficiency in Europeans and their age-dependent penetrance in multigenerational pedigrees. Diabetes 57:2511–2518 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

