Quantification of thyroglobulin, a low-abundance serum protein, by immunoaffinity peptide enrichment and tandem mass spectrometry
- PMID: 18801935
- PMCID: PMC2739673
- DOI: 10.1373/clinchem.2008.109652
Quantification of thyroglobulin, a low-abundance serum protein, by immunoaffinity peptide enrichment and tandem mass spectrometry
Abstract
Background: Quantification of serum tumor markers plays an important role in determining whether patients treated for cancer require further therapy. Whereas large-scale proteomic efforts aim to identify novel tumor markers to facilitate early detection, optimization of methods for quantifying known tumor markers offers another approach to improving management of malignancies. For example, immunoassays used in clinical practice to measure established tumor markers suffer from potential interference from endogenous immunoglobulins and imperfect concordance across platforms-problems that also plague many other immunoassays. To address these important limitations, this study used peptide immunoaffinity enrichment in concert with liquid chromatography-tandem mass spectrometry (LC-MS/MS) to quantify thyroglobulin, a well-characterized tumor marker.
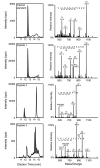
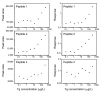
Methods: We identified 3 peptides in tryptic digests of thyroglobulin that were detected at low concentrations by tandem mass spectrometry, raised polyclonal antibodies to those peptides, and used the antibodies to extract the 3 corresponding peptides from tryptic digests of human serum. We quantified each endogenous peptide using LC-MS/MS and multiple reaction monitoring with external calibrators.
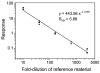
Results: The detection limit for endogenous thyroglobulin in serum was 2.6 microg/L (4 pmol/L). Direct comparison with immunoassay revealed good correlation (r(2) = 0.81).
Conclusions: Immunoaffinity peptide enrichment-tandem mass spectrometry can detect tryptic peptides of thyroglobulin at picomolar concentrations while also digesting the endogenous immunoglobulins that can potentially interfere with traditional immunoassays. Our observations suggest a general analytical strategy for using immunoaffinity isolation together with tandem mass spectrometry to quantify tumor antigens and other low-abundance proteins in human serum.
Figures
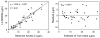
Comment in
-
Protein quantitation through targeted mass spectrometry: the way out of biomarker purgatory?Clin Chem. 2008 Nov;54(11):1749-52. doi: 10.1373/clinchem.2008.114686. Clin Chem. 2008. PMID: 18957555 Free PMC article. No abstract available.
References
-
- Rifai N, Gillette MA, Carr SA. Protein Biomarker Discovery and Validation: The Long and Uncertain Path to Clinical Utility. Nat Biotechnol. 2006;24:971–83. - PubMed
-
- Pacini F. Follow-up of Differentiated Thyroid Cancer. Eur J Nucl Med Mol Imaging. 2002;29:S492–6. - PubMed
-
- Saghari M, Gholamrezanezhad A, Mirpour S, Eftekhari M, Takavar A, Fard-Esfahani A, et al. Efficacy of Radioiodine Therapy in the Treatment of Elevated Serum Thyroglobulin in Patients with Differentiated Thyroid Carcinoma and Negative Whole-Body Iodine Scan. Nucl Med Commun. 2006;27:567–72. - PubMed
-
- Whitley RJ, Ain KB. Thyroglobulin: A Specific Serum Marker for the Management of Thyroid Carcinoma. Clin Lab Med. 2004;24:29–47. - PubMed
-
- Kloos RT, Mazzaferri EL. A Single Recombinant Human Thyrotropin-Stimulated Serum Thyroglobulin Measurement Predicts Differentiated Thyroid Carcinoma Metastases Three to Five Years Later. J Clin Endocrinol Metab. 2005;90:5047–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

