Estrogen inhibits dendritic cell maturation to RNA viruses
- PMID: 18802009
- PMCID: PMC2597128
- DOI: 10.1182/blood-2008-04-148692
Estrogen inhibits dendritic cell maturation to RNA viruses
Abstract
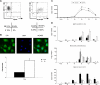
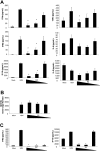
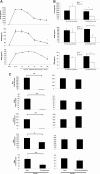
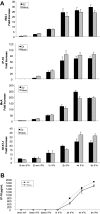
Dendritic cells (DCs) play a central role in initiating and polarizing the immune response. Therefore, DC maturation represents a key control point in the shift from innate to adaptive immunity. It is suspected that during pregnancy, hormones are critical factors that modulate changes reported to occur in maternal immunity. Here we examined the effect of 17-beta-estradiol (E2) on the maturational response triggered by virus in human DCs and its influence on their ability to activate naive T cells. We developed an in vitro system to measure the response of DCs to virus infection with Newcastle disease virus (NDV) after a 24-hour E2 treatment. Using this system, we demonstrated that E2 pretreatment down-regulated the antiviral response to RNA viruses in DCs by profoundly suppressing type I interferon (IFN) synthesis and other important inflammatory products. In addition, the DCs capacity to stimulate naive CD4 T cells was also reduced. These results suggest an important role for E2 in suppressing the antiviral response and provide a mechanism for the reduced immunity to virus infection observed during pregnancy.
Figures


References
-
- van Duivenvoorde LM, van Mierlo GJ, Boonman ZF, Toes RE. Dendritic cells: vehicles for tolerance induction and prevention of autoimmune diseases. Immunobiology. 2006;211:627–632. - PubMed
-
- Granucci F, Zanoni I, Feau S, Capuano G, Ricciardi-Castagnoli P. The regulatory role of dendritic cells in the immune response. Int Arch Allergy Immunol. 2004;134:179–185. - PubMed
-
- Wang RF, Miyahara Y, Wang HY. Toll-like receptors and immune regulation: implications for cancer therapy. Oncogene. 2008;27:181–189. - PubMed
-
- Kumagai Y, Takeuchi O, Kato H, et al. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 2007;27:240–252. - PubMed
-
- Yoneyama M, Kikuchi M, Matsumoto K, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

