Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia
- PMID: 18802023
- PMCID: PMC2729271
- DOI: 10.1161/CIRCRESAHA.108.181115
Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia
Abstract
The density of native (preexisting) collaterals and their capacity to enlarge into large conduit arteries in ischemia (arteriogenesis) are major determinants of the severity of tissue injury in occlusive disease. Mechanisms directing arteriogenesis remain unclear. Moreover, nothing is known about how native collaterals form in healthy tissue. Evidence suggests vascular endothelial growth factor (VEGF), which is important in embryonic vascular patterning and ischemic angiogenesis, may contribute to native collateral formation and arteriogenesis. Therefore, we examined mice heterozygous for VEGF receptor-1 (VEGFR-1(+/-)), VEGF receptor-2 (VEGFR-2(+/-)), and overexpressing (VEGF(hi/+)) and underexpressing VEGF-A (VEGF(lo/+)). Recovery from hindlimb ischemia was followed for 21 days after femoral artery ligation. All statements below are P<0.05. Compared to wild-type mice, VEGFR-2(+/-) showed similar: ischemic scores, recovery of hindlimb perfusion, pericollateral leukocytes, collateral enlargement, and angiogenesis. In contrast, VEGFR-1(+/-) showed impaired: perfusion recovery, pericollateral leukocytes, collateral enlargement, worse ischemic scores, and comparable angiogenesis. Compared to wild-type mice, VEGF(lo/+) had 2-fold lower perfusion immediately after ligation (suggesting fewer native collaterals which was confirmed by angiography) and blunted recovery of perfusion. VEGF(hi/+) mice had 3-fold greater perfusion immediately after ligation, more native collaterals, and improved recovery of perfusion. These differences were confirmed in the cerebral pial cortical circulation where, compared to VEGF(hi/+) mice, VEGF(lo/+) formed fewer collaterals during the perinatal period when adult density was established, and had 2-fold larger infarctions after middle cerebral artery ligation. Our findings indicate VEGF and VEGFR-1 are determinants of arteriogenesis. Moreover, we describe the first signaling molecule, VEGF-A, that specifies formation of native collaterals in healthy tissues.
Conflict of interest statement
Figures



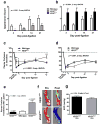
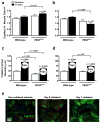
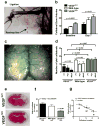
Comment in
-
Vascular endothelial growth factor and the collateral circulation: the story continues.Circ Res. 2008 Oct 24;103(9):905-6. doi: 10.1161/01.RES.0000338258.90706.2c. Circ Res. 2008. PMID: 18948626 Free PMC article. No abstract available.
Similar articles
-
Vascular endothelial growth factor and the collateral circulation: the story continues.Circ Res. 2008 Oct 24;103(9):905-6. doi: 10.1161/01.RES.0000338258.90706.2c. Circ Res. 2008. PMID: 18948626 Free PMC article. No abstract available.
-
P2Y2 nucleotide receptor mediates arteriogenesis in a murine model of hind limb ischemia.J Vasc Surg. 2016 Jan;63(1):216-25. doi: 10.1016/j.jvs.2014.06.112. Epub 2014 Jul 31. J Vasc Surg. 2016. PMID: 25088742 Free PMC article.
-
Mechanisms of Amplified Arteriogenesis in Collateral Artery Segments Exposed to Reversed Flow Direction.Arterioscler Thromb Vasc Biol. 2015 Nov;35(11):2354-65. doi: 10.1161/ATVBAHA.115.305775. Epub 2015 Sep 3. Arterioscler Thromb Vasc Biol. 2015. PMID: 26338297 Free PMC article.
-
The chemokine system in arteriogenesis and hind limb ischemia.J Vasc Surg. 2007 Jun;45 Suppl A(Suppl A):A48-56. doi: 10.1016/j.jvs.2007.02.030. J Vasc Surg. 2007. PMID: 17544024 Free PMC article. Review.
-
Collateral blood vessels in stroke and ischemic disease: Formation, physiology, rarefaction, remodeling.J Cereb Blood Flow Metab. 2025 Jun;45(6):1007-1030. doi: 10.1177/0271678X251322378. Epub 2025 Mar 12. J Cereb Blood Flow Metab. 2025. PMID: 40072222 Free PMC article. Review.
Cited by
-
Vascular Regeneration in Ischemic Hindlimb by Adeno-Associated Virus Expressing Conditionally Silenced Vascular Endothelial Growth Factor.J Am Heart Assoc. 2016 May 26;5(6):e001815. doi: 10.1161/JAHA.115.001815. J Am Heart Assoc. 2016. PMID: 27231018 Free PMC article.
-
Expounding newer vistas in VEGF.Indian J Ophthalmol. 2013 Jan-Feb;61(1):1-2. doi: 10.4103/0301-4738.105047. Indian J Ophthalmol. 2013. PMID: 23275212 Free PMC article. No abstract available.
-
Plasma microRNA-210 is associated with VEGF-A and EphrinA3 and relates to coronary collateral circulation in patients with coronary heart disease: a cross-sectional study.BMC Cardiovasc Disord. 2025 Jul 28;25(1):547. doi: 10.1186/s12872-025-05013-y. BMC Cardiovasc Disord. 2025. PMID: 40717103 Free PMC article.
-
Regional leptomeningeal score on CT angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions.AJNR Am J Neuroradiol. 2011 Oct;32(9):1640-5. doi: 10.3174/ajnr.A2564. Epub 2011 Jul 28. AJNR Am J Neuroradiol. 2011. PMID: 21799045 Free PMC article.
-
BAG3 (Bcl-2-Associated Athanogene-3) Coding Variant in Mice Determines Susceptibility to Ischemic Limb Muscle Myopathy by Directing Autophagy.Circulation. 2017 Jul 18;136(3):281-296. doi: 10.1161/CIRCULATIONAHA.116.024873. Epub 2017 Apr 25. Circulation. 2017. PMID: 28442482 Free PMC article.
References
-
- Grundmann S, Piek JJ, Pasterkamp G, Hoefer IE. Arteriogenesis: basic mechanisms and therapeutic stimulation. Eur J Clin Invest. 2007;37:755–66. - PubMed
-
- Sherman JA, Hall A, Malenka DJ, De Muinck ED, Simons M. Humoral and cellular factors responsible for coronary collateral formation. Am J Cardiol. 2006;98:1194–7. - PubMed
-
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. - PubMed
-
- von Degenfeld G, Banfi A, Springer ML, Wagner RA, Jacobi J, Ozawa CR, Merchant MJ, Cooke JP, Blau HM. Microenvironmental VEGF distribution is critical for stable and functional vessel growth in ischemia. Faseb J. 2006;20:2657–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

