Disruption of sarcolemmal ATP-sensitive potassium channel activity impairs the cardiac response to systolic overload
- PMID: 18802029
- PMCID: PMC2877276
- DOI: 10.1161/CIRCRESAHA.107.170795
Disruption of sarcolemmal ATP-sensitive potassium channel activity impairs the cardiac response to systolic overload
Abstract
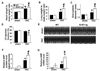
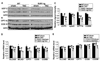
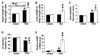
Sarcolemmal ATP-sensitive potassium channels (K(ATP)) act as metabolic sensors that facilitate adaptation of the left ventricle to changes in energy requirements. This study examined the mechanism by which K(ATP) dysfunction impairs the left ventricular response to stress using transgenic mouse strains with cardiac-specific disruption of K(ATP) activity (SUR1-tg mice) or Kir6.2 gene deficiency (Kir6.2 KO). Both SUR1-tg and Kir6.2 KO mice had normal left ventricular mass and function under unstressed conditions. Following chronic transverse aortic constriction, both SUR1-tg and Kir6.2 KO mice developed more severe left ventricular hypertrophy and dysfunction as compared with their corresponding WT controls. Both SUR1-tg and Kir6.2 KO mice had significantly decreased expression of peroxisome proliferator-activated receptor gamma coactivator (PGC)-1alpha and a group of energy metabolism related genes at both protein and mRNA levels. Furthermore, disruption of K(ATP) repressed expression and promoter activity of PGC-1alpha in cultured rat neonatal cardiac myocytes in response to hypoxia, indicating that K(ATP) activity is required to maintain PGC-1alpha expression under stress conditions. PGC-1alpha gene deficiency also exacerbated chronic transverse aortic constriction-induced ventricular hypertrophy and dysfunction, suggesting that depletion of PGC-1alpha can worsen systolic overload induced ventricular dysfunction. Both SUR1-tg and Kir6.2 KO mice had decreased FOXO1 after transverse aortic constriction, in agreement with the reports that a decrease of FOXO1 can repress PGC-1alpha expression. Furthermore, inhibition of K(ATP) caused a decrease of FOXO1 associated with PGC-1alpha promoter. These data indicate that K(ATP) channels facilitate the cardiac response to stress by regulating PGC-1alpha and its target genes, at least partially through the FOXO1 pathway.
Figures


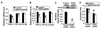



Similar articles
-
Diazoxide maintenance of myocyte volume and contractility during stress: evidence for a non-sarcolemmal K(ATP) channel location.J Thorac Cardiovasc Surg. 2010 Nov;140(5):1153-9. doi: 10.1016/j.jtcvs.2010.07.047. J Thorac Cardiovasc Surg. 2010. PMID: 20804990 Free PMC article.
-
HMR 1098 is not an SUR isotype specific inhibitor of heterologous or sarcolemmal K ATP channels.J Mol Cell Cardiol. 2011 Mar;50(3):552-60. doi: 10.1016/j.yjmcc.2010.12.011. Epub 2010 Dec 23. J Mol Cell Cardiol. 2011. PMID: 21185839 Free PMC article.
-
Transgenic overexpression of SUR1 in the heart suppresses sarcolemmal K(ATP).J Mol Cell Cardiol. 2005 Oct;39(4):647-56. doi: 10.1016/j.yjmcc.2005.06.003. J Mol Cell Cardiol. 2005. PMID: 16099470
-
Cardiac sarcolemmal K(ATP) channels: Latest twists in a questing tale!J Mol Cell Cardiol. 2010 Jan;48(1):71-5. doi: 10.1016/j.yjmcc.2009.07.002. Epub 2009 Jul 14. J Mol Cell Cardiol. 2010. PMID: 19607836 Free PMC article. Review.
-
Sulfonylurea receptor 1 subunits of ATP-sensitive potassium channels and myocardial ischemia/reperfusion injury.Trends Cardiovasc Med. 2009 Feb;19(2):61-7. doi: 10.1016/j.tcm.2009.04.008. Trends Cardiovasc Med. 2009. PMID: 19577714 Free PMC article. Review.
Cited by
-
Single-Channel Properties of the ROMK-Pore-Forming Subunit of the Mitochondrial ATP-Sensitive Potassium Channel.Int J Mol Sci. 2019 Oct 25;20(21):5323. doi: 10.3390/ijms20215323. Int J Mol Sci. 2019. PMID: 31731540 Free PMC article.
-
lncRNA Gm20257 alleviates pathological cardiac hypertrophy by modulating the PGC-1α-mitochondrial complex IV axis.Front Med. 2024 Aug;18(4):664-677. doi: 10.1007/s11684-024-1065-7. Epub 2024 Jun 27. Front Med. 2024. PMID: 38926249
-
Preconditioning by isoflurane elicits mitochondrial protective mechanisms independent of sarcolemmal KATP channel in mouse cardiomyocytes.J Cardiovasc Pharmacol. 2013 May;61(5):369-77. doi: 10.1097/FJC.0b013e318285f55b. J Cardiovasc Pharmacol. 2013. PMID: 23318991 Free PMC article.
-
KATP channels and cardiovascular disease: suddenly a syndrome.Circ Res. 2013 Mar 29;112(7):1059-72. doi: 10.1161/CIRCRESAHA.112.300514. Circ Res. 2013. PMID: 23538276 Free PMC article. Review.
-
Decreased gene expression of KACh and KATP channels in hyperthyroid rabbit atria.Int J Clin Exp Pathol. 2022 Mar 15;15(3):145-151. eCollection 2022. Int J Clin Exp Pathol. 2022. PMID: 35414842 Free PMC article.
References
-
- Miki T, Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. Journal of Molecular and Cellular Cardiology. 2005;38:917–925. - PubMed
-
- Cui N, Li L, Wang X, Shi Y, Shi W, Jiang C. Elimination of allosteric modulation of myocardial KATP channels by ATP and protons in two Kir6.2 polymorphisms found in sudden cardiac death. Physiol Genomics. 2006;25:105–115. - PubMed
-
- Kane GC, Behfar A, Yamada S, Perez-Terzic C, O'Cochlain F, Reyes S, Dzeja PP, Miki T, Seino S, Terzic A. ATP-Sensitive K+ Channel Knockout Compromises the Metabolic Benefit of Exercise Training, Resulting in Cardiac Deficits. Diabetes. 2004;53:S169–S175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

