Characterization of the steric defense of the HIV-1 gp41 N-trimer region
- PMID: 18802030
- PMCID: PMC2590922
- DOI: 10.1110/ps.038273.108
Characterization of the steric defense of the HIV-1 gp41 N-trimer region
Abstract
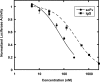
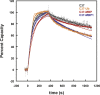
During viral entry, HIV gp41 adopts a transient conformation called the "prehairpin intermediate" in which a highly conserved therapeutic target, the N-trimer, is exposed. Despite extensive discovery efforts, potent and broadly neutralizing antibodies that target the N-trimer are elusive. We previously demonstrated the N-trimer is protected by a steric block that prevents large proteins, such as antibodies, from accessing it. Here we further characterize the steric block and identify its source. To study the N-trimer steric accessibility, we produced two sets of C-peptide inhibitors (a potent inhibitor targeting the N-trimer) fused to cargo proteins of increasing size facing either the virus or cell side of the prehairpin intermediate. Both bulky inhibitor sets show a steric block, but the effect is more pronounced with virus-side cargo. Additionally, both sets maintain their potencies in a modified entry assay that removes possible sources of target cell steric hindrance. These results implicate a viral source, likely gp120, as the primary component of the steric block. In addition, we studied the steric accessibility of the "pocket" region of the N-trimer, a highly attractive drug and vaccine target. We demonstrated a pocket-specific antibody, D5, is more potent as an scFv than as a full-length IgG, suggesting the N-trimer steric restriction extends to the pocket. This characterization will facilitate the design of sterically restricted antigens that mimic the steric environment of the N-trimer in the prehairpin intermediate and are capable of inducing potent and broadly neutralizing antibodies that circumvent the N-trimer steric block.
Figures



Similar articles
-
Steric accessibility of the HIV-1 gp41 N-trimer region.J Biol Chem. 2005 Apr 1;280(13):12567-72. doi: 10.1074/jbc.M412770200. Epub 2005 Jan 18. J Biol Chem. 2005. PMID: 15657041
-
Structural basis for HIV-1 neutralization by a gp41 fusion intermediate-directed antibody.Nat Struct Mol Biol. 2006 Aug;13(8):740-7. doi: 10.1038/nsmb1127. Epub 2006 Jul 23. Nat Struct Mol Biol. 2006. PMID: 16862157
-
Small molecules that bind the inner core of gp41 and inhibit HIV envelope-mediated fusion.Proc Natl Acad Sci U S A. 2006 Sep 19;103(38):13938-43. doi: 10.1073/pnas.0601036103. Epub 2006 Sep 8. Proc Natl Acad Sci U S A. 2006. PMID: 16963566 Free PMC article.
-
Short communication: In vitro synergy between peptides or neutralizing antibodies targeting the N- and C-terminal heptad repeats of HIV Type 1 gp41.AIDS Res Hum Retroviruses. 2008 Dec;24(12):1537-44. doi: 10.1089/aid.2008.0129. AIDS Res Hum Retroviruses. 2008. PMID: 19102685 Review.
-
Viral surface glycoproteins, gp120 and gp41, as potential drug targets against HIV-1: brief overview one quarter of a century past the approval of zidovudine, the first anti-retroviral drug.Eur J Med Chem. 2011 Apr;46(4):979-92. doi: 10.1016/j.ejmech.2011.01.046. Epub 2011 Feb 3. Eur J Med Chem. 2011. PMID: 21345545 Review.
Cited by
-
Long-lasting enfuvirtide carrier pentasaccharide conjugates with potent anti-human immunodeficiency virus type 1 activity.Antimicrob Agents Chemother. 2010 Jan;54(1):134-42. doi: 10.1128/AAC.00827-09. Epub 2009 Oct 5. Antimicrob Agents Chemother. 2010. PMID: 19805567 Free PMC article.
-
A Novel gp41-Binding Adnectin with Potent Anti-HIV Activity Is Highly Synergistic when Linked to a CD4-Binding Adnectin.J Virol. 2018 Jun 29;92(14):e00421-18. doi: 10.1128/JVI.00421-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743355 Free PMC article.
-
Affinity maturation by targeted diversification of the CDR-H2 loop of a monoclonal Fab derived from a synthetic naïve human antibody library and directed against the internal trimeric coiled-coil of gp41 yields a set of Fabs with improved HIV-1 neutralization potency and breadth.Virology. 2009 Oct 10;393(1):112-9. doi: 10.1016/j.virol.2009.07.019. Epub 2009 Aug 19. Virology. 2009. PMID: 19695655 Free PMC article.
-
A Derivative of the D5 Monoclonal Antibody That Targets the gp41 N-Heptad Repeat of HIV-1 with Broad Tier-2-Neutralizing Activity.J Virol. 2021 Jul 12;95(15):e0235020. doi: 10.1128/JVI.02350-20. Epub 2021 Jul 12. J Virol. 2021. PMID: 33980592 Free PMC article.
-
Receptor Activation of HIV-1 Env Leads to Asymmetric Exposure of the gp41 Trimer.PLoS Pathog. 2016 Dec 19;12(12):e1006098. doi: 10.1371/journal.ppat.1006098. eCollection 2016 Dec. PLoS Pathog. 2016. PMID: 27992602 Free PMC article.
References
-
- Binley J.M., Sanders R.W., Clas B., Schuelke N., Master A., Guo Y., Kajumo F., Anselma D.J., Maddon P.J., Olson W.C., et al. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 2000;74:627–643. - PMC - PubMed
-
- Chan D.C., Fass D., Berger J.M., Kim P.S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. - PubMed
-
- Choe H., Li W., Wright P.L., Vasilieva N., Venturi M., Huang C.C., Grundner C., Dorfman T., Zwick M.B., Wang L., et al. Tyrosine sulfation of human antibodies contributes to recognition of the CCR5 binding region of HIV-1 gp120. Cell. 2003;114:161–170. - PubMed
-
- Connor R.I., Chen B.K., Choe S., Landau N.R. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

