Prospective isolation of skeletal muscle stem cells with a Pax7 reporter
- PMID: 18802040
- PMCID: PMC4372243
- DOI: 10.1634/stemcells.2007-1017
Prospective isolation of skeletal muscle stem cells with a Pax7 reporter
Abstract
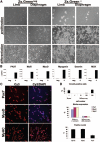
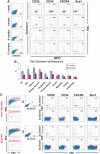
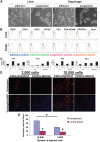
Muscle regeneration occurs through activation of quiescent satellite cells whose progeny proliferate, differentiate, and fuse to make new myofibers. We used a transgenic Pax7-ZsGreen reporter mouse to prospectively isolate stem cells of skeletal muscle by flow cytometry. We show that Pax7-expressing cells (satellite cells) in the limb, head, and diaphragm muscles are homogeneous in size and granularity and uniformly labeled by certain cell surface markers, including CD34 and CD29. The frequency of the satellite cells varies between muscle types and with age. Clonal analysis demonstrated that all colonies arising from single cells within the Pax7-sorted fraction have myogenic potential. In response to injury, Pax7(+) cells reduce CD34, CD29, and CXCR4 expression, increase in size, and acquire Sca-1. When directly isolated and cultured in vitro, Pax7(+) cells display the hallmarks of activation and proliferate, initially as suspension aggregates and later distributed between suspension and adherence. During in vitro expansion, Pax7 (ZsGreen) and CD34 expression decline, whereas expression of PSA-NCAM is acquired. The nonmyogenic, Pax7(neg) cells expand as Sca1(+) PDGRalpha(+) PSA-NCAM(neg) cells. Satellite cells expanded exclusively in suspension can engraft and produce dystrophin(+) fibers in mdx(-/-) mice. These results establish a novel animal model for the study of muscle stem cell physiology and a culture system for expansion of engraftable muscle progenitors.
Figures



References
-
- Schultz E. Satellite cells in normal, regenerating and dystrophic muscle. Adv Exp Med Biol. 1985;182:73–84. - PubMed
-
- Bischoff R. A satellite cell mitogen from crushed adult muscle. Dev Biol. 1986;115:140–147. - PubMed
-
- Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: An EM and radioautographic study. J Exp Zool. 1978;206:451–456. - PubMed
-
- Snow MH. A quantitative ultrastructural analysis of satellite cells in denervated fast and slow muscles of the mouse. Anat Rec. 1983;207:593–604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

