Transplantation survival is maintained by granzyme B+ regulatory cells and adaptive regulatory T cells
- PMID: 18802078
- PMCID: PMC2572718
- DOI: 10.4049/jimmunol.181.7.4752
Transplantation survival is maintained by granzyme B+ regulatory cells and adaptive regulatory T cells
Abstract
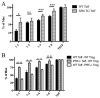
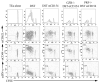
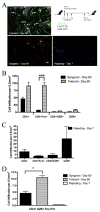
Granzyme B (GZB) has been implicated as an effector mechanism in regulatory T cells (T(reg)) suppression. In a model of T(reg)-dependent graft tolerance, it is shown that GZB- deficient mice are unable to establish long-term tolerance. Moreover, mice overexpressing the inhibitor of GZB, serine protease inhibitor 6, are also resistant to tolerization to alloantigen. Graft survival was shorter in bone marrow-mixed chimeras reconstituted with GZB-deficient T(reg) as compared with wild-type T(reg). Whereas there was no difference in graft survival in mixed chimeras reconstituted with wild-type, perforin-deficient, or Fas ligand-deficient T(reg). Finally, data also show that if alloreactive effectors cannot express FoxP3 and be induced to convert in the presence of competent T(reg), then graft tolerance is lost. Our data are the first in vivo data to implicate GZB expression by T(reg) in sustaining long-lived graft survival.
Conflict of interest statement
Figures


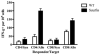
Similar articles
-
Granzyme B is not required for regulatory T cell-mediated suppression of graft-versus-host disease.Blood. 2010 Mar 4;115(9):1669-77. doi: 10.1182/blood-2009-07-233676. Epub 2009 Nov 13. Blood. 2010. PMID: 19965675 Free PMC article.
-
Increased CD4+Foxp3+ T cells in BAFF-transgenic mice suppress T cell effector responses.J Immunol. 2009 Jan 15;182(2):793-801. doi: 10.4049/jimmunol.182.2.793. J Immunol. 2009. PMID: 19124722
-
Chimeric acceleration by donor CD4+CD25+T-reg depleted fraction in splenocyte transplantation.J Surg Res. 2012 Nov;178(1):133-8. doi: 10.1016/j.jss.2012.01.003. Epub 2012 Apr 1. J Surg Res. 2012. PMID: 22502904
-
Serine protease inhibitor-6 differentially affects the survival of effector and memory alloreactive CD8-T cells.Am J Transplant. 2015 Jan;15(1):234-41. doi: 10.1111/ajt.13051. Am J Transplant. 2015. PMID: 25534448 Free PMC article.
-
Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells.J Exp Med. 2013 Feb 11;210(2):257-68. doi: 10.1084/jem.20121525. Epub 2013 Feb 4. J Exp Med. 2013. PMID: 23382542 Free PMC article.
Cited by
-
Function and Role of Regulatory T Cells in Rheumatoid Arthritis.Front Immunol. 2021 Apr 1;12:626193. doi: 10.3389/fimmu.2021.626193. eCollection 2021. Front Immunol. 2021. PMID: 33868244 Free PMC article. Review.
-
The calcineurin inhibitor tacrolimus allows the induction of functional CD4CD25 regulatory T cells by rabbit anti-thymocyte globulins.Clin Exp Immunol. 2010 Aug;161(2):364-77. doi: 10.1111/j.1365-2249.2010.04183.x. Epub 2010 May 28. Clin Exp Immunol. 2010. PMID: 20528886 Free PMC article.
-
Cytolytic CD4+ and CD8+ Regulatory T-Cells and Implications for Developing Immunotherapies to Combat Graft-Versus-Host Disease.Front Immunol. 2022 Apr 12;13:864748. doi: 10.3389/fimmu.2022.864748. eCollection 2022. Front Immunol. 2022. PMID: 35493508 Free PMC article. Review.
-
Mechanisms of Tolerance Induction by Hematopoietic Chimerism: The Immune Perspective.Stem Cells Transl Med. 2017 Mar;6(3):700-712. doi: 10.1002/sctm.16-0358. Epub 2017 Jan 3. Stem Cells Transl Med. 2017. PMID: 28186688 Free PMC article. Review.
-
Selective expansion of regulatory T cells using an orthogonal IL-2/IL-2 receptor system facilitates transplantation tolerance.J Clin Invest. 2021 Apr 15;131(8):e139991. doi: 10.1172/JCI139991. J Clin Invest. 2021. PMID: 33855972 Free PMC article.
References
-
- Billingham B, Brent L, Medawar P. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. - PubMed
-
- Burnet F. Self and Not-Self. Cambridge Univ. Press; Cambridge, MA: 1969.
-
- Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. - PubMed
-
- Jones ND, Van Maurik A, Hara M, Spriewald BM, Witzke O, Morris PJ, Wood KJ. CD40-CD40 ligand-independent activation of CD8+ T cells can trigger allograft rejection. J Immunol. 2000;165:1111–1118. - PubMed
-
- Jarvinen LZ, Blazar BR, Adeyi OA, Strom TB, Noelle RJ. CD154 on the surface of CD4+CD25+ regulatory T cells contributes to skin transplant tolerance. Transplantation. 2003;76:1375–1379. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

