Protein kinase C theta regulates stability of the peripheral adhesion ring junction and contributes to the sensitivity of target cell lysis by CTL
- PMID: 18802085
- PMCID: PMC2748977
- DOI: 10.4049/jimmunol.181.7.4815
Protein kinase C theta regulates stability of the peripheral adhesion ring junction and contributes to the sensitivity of target cell lysis by CTL
Abstract
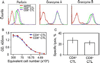
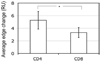
Destruction of virus-infected cells by CTL is an extremely sensitive and efficient process. Our previous data suggest that LFA-1-ICAM-1 interactions in the peripheral supramolecular activation cluster (pSMAC) of the immunological synapse mediate formation of a tight adhesion junction that might contribute to the sensitivity of target cell lysis by CTL. Herein, we compared more (CD8(+)) and less (CD4(+)) effective CTL to understand the molecular events that promote efficient target cell lysis. We found that abrogation of the pSMAC formation significantly impaired the ability of CD8(+) but not CD4(+) CTL to lyse target cells despite having no effect of the amount of released granules by both CD8(+) and CD4(+) CTL. Consistent with this, CD4(+) CTL break their synapses more often than do CD8(+) CTL, which leads to the escape of the cytolytic molecules from the interface. CD4(+) CTL treatment with a protein kinase Ctheta inhibitor increases synapse stability and sensitivity of specific target cell lysis. Thus, formation of a stable pSMAC, which is partially controlled by protein kinase Ctheta, functions to confine the released lytic molecules at the synaptic interface and to enhance the effectiveness of target cell lysis.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures








Similar articles
-
CD2/LFA-3 or LFA-1/ICAM-1 but not CD28/B7 interactions can augment cytotoxicity by virus-specific CD8+ cytotoxic T lymphocytes.Eur J Immunol. 1993 Feb;23(2):418-24. doi: 10.1002/eji.1830230218. Eur J Immunol. 1993. PMID: 7679643
-
Comparative clonal analysis of human immunodeficiency virus type 1 (HIV-1)-specific CD4+ and CD8+ cytolytic T lymphocytes isolated from seronegative humans immunized with candidate HIV-1 vaccines.J Exp Med. 1992 Dec 1;176(6):1531-42. doi: 10.1084/jem.176.6.1531. J Exp Med. 1992. PMID: 1460417 Free PMC article. Clinical Trial.
-
CD4+ T cell-induced differentiation of EBV-transformed lymphoblastoid cells is associated with diminished recognition by EBV-specific CD8+ cytotoxic T cells.J Immunol. 2003 Mar 15;170(6):3187-94. doi: 10.4049/jimmunol.170.6.3187. J Immunol. 2003. PMID: 12626577
-
Interplay between the TCR/CD3 complex and CD4 or CD8 in the activation of cytotoxic T lymphocytes.Immunol Rev. 1989 Jun;109:119-41. doi: 10.1111/j.1600-065x.1989.tb00022.x. Immunol Rev. 1989. PMID: 2527803 Review.
-
Induction of lytic pathways in T cell clones derived from wild-type or protein tyrosine kinase Fyn mutant mice.Immunol Rev. 1995 Aug;146:117-44. doi: 10.1111/j.1600-065x.1995.tb00687.x. Immunol Rev. 1995. PMID: 7493751 Review.
Cited by
-
Limited immune surveillance in lymphoid tissue by cytolytic CD4+ T cells during health and HIV disease.PLoS Pathog. 2018 Apr 13;14(4):e1006973. doi: 10.1371/journal.ppat.1006973. eCollection 2018 Apr. PLoS Pathog. 2018. Retraction in: PLoS Pathog. 2025 Aug 27;21(8):e1013442. doi: 10.1371/journal.ppat.1013442. PMID: 29652923 Free PMC article. Retracted.
-
HIV Envelope gp120 Alters T Cell Receptor Mobilization in the Immunological Synapse of Uninfected CD4 T Cells and Augments T Cell Activation.J Virol. 2016 Nov 14;90(23):10513-10526. doi: 10.1128/JVI.01532-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27630246 Free PMC article.
-
The immunological synapse.Cancer Immunol Res. 2014 Nov;2(11):1023-33. doi: 10.1158/2326-6066.CIR-14-0161. Cancer Immunol Res. 2014. PMID: 25367977 Free PMC article. Review.
-
The immune synapses reveal aberrant functions of CD8 T cells during chronic HIV infection.Nat Commun. 2022 Oct 28;13(1):6436. doi: 10.1038/s41467-022-34157-0. Nat Commun. 2022. PMID: 36307445 Free PMC article.
-
Understanding the structure and function of the immunological synapse.Cold Spring Harb Perspect Biol. 2010 Oct;2(10):a002311. doi: 10.1101/cshperspect.a002311. Epub 2010 Sep 15. Cold Spring Harb Perspect Biol. 2010. PMID: 20843980 Free PMC article. Review.
References
-
- Mentzer SJ, Smith BR, Barbosa JA, Crimmins MA, Herrmann SH, Burakoff SJ. CTL adhesion and antigen recognition are discrete steps in the human CTL-target cell interaction. J. Immunol. 1987;138:1325–1330. - PubMed
-
- Shaw S, Luce GE, Quinones R, Gress RE, Springer TA, Sanders ME. Two antigen-independent adhesion pathways used by human cytotoxic T-cell clones. Nature. 1986;323:262–264. - PubMed
-
- Spitz H, van Schooten W, Keizer H, van Seventer G, van de Rijn M, Terhorst C, de Vries J-E. Alloantigen recognition is preceded by nonspecific adhesion of cytotoxic T cells and target cells. Science. 1986;232:403–405. - PubMed
-
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. - PubMed
-
- Monks C, Freiberg B, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

