T cell-dendritic cell immunological synapses contain TCR-dependent CD28-CD80 clusters that recruit protein kinase C theta
- PMID: 18802089
- PMCID: PMC2556893
- DOI: 10.4049/jimmunol.181.7.4852
T cell-dendritic cell immunological synapses contain TCR-dependent CD28-CD80 clusters that recruit protein kinase C theta
Abstract
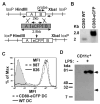
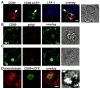
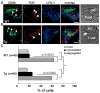
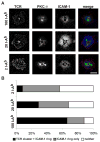
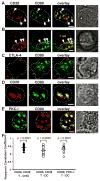
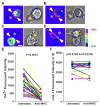
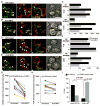
Short-lived TCR microclusters and a longer-lived protein kinase Ctheta-focusing central supramolecular activation cluster (cSMAC) have been defined in model immunological synapses (IS). In different model systems, CD28-mediated costimulatory interactions have been detected in microclusters, the cSMAC, or segregated from the TCR forming multiple distinct foci. The relationship between TCR and costimulatory molecules in the physiological IS of T cell-dendritic cell (DC) is obscure. To study the dynamic relationship of CD28-CD80 and TCR interactions in the T cell-DC IS during Ag-specific T cell activation, we generated CD80-eCFP mice using bacterial artificial chromosome transgenic technology. In splenic DCs, endogenous CD80 and CD80-eCFP localized to plasma membrane and Golgi apparatus, and CD80-eCFP was functional in vivo. In the OT-II T cell-DC IS, multiple segregated TCR, CD80, and LFA-1 clusters were detected. In the T cell-DC synapse CD80 clusters were colocalized with CD28 and PKCtheta, a characteristic of the cSMAC. Acute blockade of TCR signaling with anti-MHC Ab resulted in a rapid reduction in Ca(2+) signaling and the number and size of the CD80 clusters, a characteristic of TCR microclusters. Thus, the T cell-DC interface contains dynamic costimulatory foci that share characteristics of microclusters and cSMACs.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

