CD8 T cells utilize TRAIL to control influenza virus infection
- PMID: 18802095
- PMCID: PMC2610351
- DOI: 10.4049/jimmunol.181.7.4918
CD8 T cells utilize TRAIL to control influenza virus infection
Erratum in
- J Immunol. 2008 Nov 15;181(10)7428
Abstract
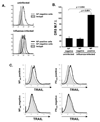
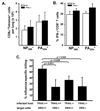
Elimination of influenza virus-infected cells during primary influenza virus infections is thought to be mediated by CD8(+) T cells though perforin- and FasL-mediated mechanisms. However, recent studies suggest that CD8(+) T cells can also utilize TRAIL to kill virally infected cells. Therefore, we herein examined the importance of TRAIL to influenza-specific CD8(+) T cell immunity and to the control of influenza virus infections. Our results show that TRAIL deficiency increases influenza-associated morbidity and influenza virus titers, and that these changes in disease severity are coupled to decreased influenza-specific CD8(+) T cell cytotoxicity in TRAIL(-/-) mice, a decrease that occurs despite equivalent numbers of pulmonary influenza-specific CD8(+) T cells. Furthermore, TRAIL expression occurs selectively on influenza-specific CD8(+) T cells, and high TRAIL receptor (DR5) expression occurs selectively on influenza virus-infected pulmonary epithelial cells. Finally, we show that adoptive transfer of TRAIL(+/+) but not TRAIL(-/-) CD8(+) effector T cells alters the mortality associated with lethal dose influenza virus infections. Collectively, our results suggest that TRAIL is an important component of immunity to influenza infections and that TRAIL deficiency decreases CD8(+) T cell-mediated cytotoxicity, leading to more severe influenza infections.
Conflict of interest statement
The authors have no financial conflict of interest
Figures




References
-
- Doherty PC. Cytotoxic T cell effector and memory function in viral immunity. Curr Top Microbiol Immunol. 1996;206:1–14. - PubMed
-
- Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. - PubMed
-
- Epstein SL, Lo CY, Misplon JA, Bennink JR. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J Immunol. 1998;160:322–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

