Annexin A1 regulates intestinal mucosal injury, inflammation, and repair
- PMID: 18802107
- PMCID: PMC2778483
- DOI: 10.4049/jimmunol.181.7.5035
Annexin A1 regulates intestinal mucosal injury, inflammation, and repair
Abstract
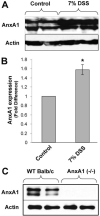
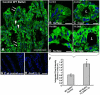
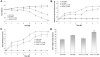
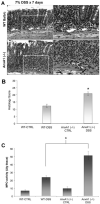
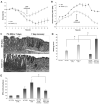
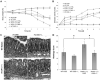
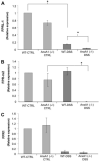
During mucosal inflammation, a complex array of proinflammatory and protective mechanisms regulates inflammation and severity of injury. Secretion of anti-inflammatory mediators is a mechanism that is critical in controlling inflammatory responses and promoting epithelial restitution and barrier recovery. AnxA1 is a potent anti-inflammatory protein that has been implicated to play a critical immune regulatory role in models of inflammation. Although AnxA1 has been shown to be secreted in intestinal mucosal tissues during inflammation, its potential role in modulating the injury/inflammatory response is not understood. In this study, we demonstrate that AnxA1-deficient animals exhibit increased susceptibility to dextran sulfate sodium (DSS)-induced colitis with greater clinical morbidity and histopathologic mucosal injury. Furthermore, impaired recovery following withdrawal of DSS administration was observed in AnxA1 (-/-) animals compared with wild-type (WT) control mice that was independent of inflammatory cell infiltration. Since AnxA1 exerts its anti-inflammatory properties through stimulation of ALX/FPRL-1, we explored the role of this receptor-ligand interaction in regulating DSS-induced colitis. Interestingly, treatment with an ALX/FPRL-1 agonist, 15-epi-lipoxin A4 reversed the enhanced sensitivity of AnxA1 (-/-) mice to DSS colitis. In contrast, 15-epi-lipoxin A4 did not significantly improve the severity of disease in WT animals. Additionally, differential expression of ALX/FPLR-1 in control and DSS-treated WT and AnxA1-deficient animals suggested a potential role for AnxA1 in regulating ALX/FPRL-1 expression under pathophysiological conditions. Together, these results support a role of endogenous AnxA1 in the protective and reparative properties of the intestinal mucosal epithelium.
Figures
Similar articles
-
Role of the protein annexin A1 on the efficacy of anti-TNF treatment in a murine model of acute colitis.Biochem Pharmacol. 2016 Sep 1;115:104-13. doi: 10.1016/j.bcp.2016.06.012. Epub 2016 Jun 22. Biochem Pharmacol. 2016. PMID: 27343762
-
The ANXA1 released from intestinal epithelial cells alleviate DSS-induced colitis by improving NKG2A expression of Natural Killer cells.Biochem Biophys Res Commun. 2016 Sep 9;478(1):213-220. doi: 10.1016/j.bbrc.2016.07.066. Epub 2016 Jul 18. Biochem Biophys Res Commun. 2016. PMID: 27435504
-
Lack of TNFRI signaling enhances annexin A1 biological activity in intestinal inflammation.Biochem Pharmacol. 2015 Dec 1;98(3):422-31. doi: 10.1016/j.bcp.2015.09.009. Epub 2015 Sep 16. Biochem Pharmacol. 2015. PMID: 26386311
-
Annexin A1 and resolution of inflammation: tissue repairing properties and signalling signature.Biol Chem. 2016 Oct 1;397(10):981-93. doi: 10.1515/hsz-2016-0200. Biol Chem. 2016. PMID: 27447237 Review.
-
Annexin A1 and glucocorticoids as effectors of the resolution of inflammation.Nat Rev Immunol. 2009 Jan;9(1):62-70. doi: 10.1038/nri2470. Nat Rev Immunol. 2009. PMID: 19104500 Review.
Cited by
-
Myeloid Cell Hypoxia-Inducible Factors Promote Resolution of Inflammation in Experimental Colitis.Front Immunol. 2018 Nov 5;9:2565. doi: 10.3389/fimmu.2018.02565. eCollection 2018. Front Immunol. 2018. PMID: 30455703 Free PMC article.
-
Targeting neutrophil apoptosis for enhancing the resolution of inflammation.Cells. 2013 May 22;2(2):330-48. doi: 10.3390/cells2020330. Cells. 2013. PMID: 24709704 Free PMC article.
-
Therapeutic Potential of Annexins in Sepsis and COVID-19.Front Pharmacol. 2021 Sep 9;12:735472. doi: 10.3389/fphar.2021.735472. eCollection 2021. Front Pharmacol. 2021. PMID: 34566657 Free PMC article. Review.
-
Plasmin and plasminogen induce macrophage reprogramming and regulate key steps of inflammation resolution via annexin A1.Blood. 2017 May 25;129(21):2896-2907. doi: 10.1182/blood-2016-09-742825. Epub 2017 Mar 20. Blood. 2017. PMID: 28320709 Free PMC article.
-
Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair.J Clin Invest. 2013 Jan;123(1):443-54. doi: 10.1172/JCI65831. Epub 2012 Dec 17. J Clin Invest. 2013. PMID: 23241962 Free PMC article.
References
-
- Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm. Bowel Dis. 2001;7:68–77. - PubMed
-
- Martin GR, Wallace JL. Gastrointestinal inflammation: a central component of mucosal defense and repair. Exp. Biol. Med. 2006;231:130–137. - PubMed
-
- Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–933. - PubMed
-
- Markossian S, Kreydiyyeh SI. TNF-α down-regulates the Na+-K+ ATPase and the Na+-K+-2Cl-cotransporter in the rat colon via PGE2. Cytokine. 2005;30:319–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 DK074706/DK/NIDDK NIH HHS/United States
- R29 DK055679/DK/NIDDK NIH HHS/United States
- T32 GM008169/GM/NIGMS NIH HHS/United States
- R01-DK61379/DK/NIDDK NIH HHS/United States
- R01 DK072564/DK/NIDDK NIH HHS/United States
- R24 DK064399/DK/NIDDK NIH HHS/United States
- R01 DK055679/DK/NIDDK NIH HHS/United States
- DK 59888/DK/NIDDK NIH HHS/United States
- K08 DK074706-01/DK/NIDDK NIH HHS/United States
- DK 55679/DK/NIDDK NIH HHS/United States
- DK64399/DK/NIDDK NIH HHS/United States
- R01 DK061379/DK/NIDDK NIH HHS/United States
- R01-DK72564/DK/NIDDK NIH HHS/United States
- T32 DK007771/DK/NIDDK NIH HHS/United States
- R01 DK059888/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

