Inflammation in acute kidney injury
- PMID: 18802372
- PMCID: PMC2614446
- DOI: 10.1159/000142934
Inflammation in acute kidney injury
Abstract
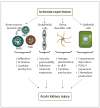
Ischemia-reperfusion injury (IRI) is one of the major causes of acute kidney injury (AKI) and evidence supporting the involvement of both innate and adaptive immunity in renal IRI has accumulated in recent years. In addition to leukocytes, kidney endothelial cells promote inflammation after IRI by increasing adhesion molecule expression and vascular permeability. Kidney tubular epithelial cells increase complement binding and upregulate toll-like receptors, both of which lead to cytokine/chemokine production in IRI. Activation of kidney resident dendritic cells, interferon-gamma-producing neutrophils, infiltrating macrophages, CD4+ T cells, B cells and invariant natural killer T cells are all implicated in the pathogenesis of AKI. The complex interplay between innate and adaptive immunity in renal IRI is still not completely understood, but major advances have been made. This review summarizes these recent advances to further our understanding of the immune mechanisms of acute kidney injury.
Copyright 2008 S. Karger AG, Basel.
Figures
References
-
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. - PubMed
-
- Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol. 2007;178:5899–5911. - PubMed
-
- Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2a receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol. 2005;288:F722–F731. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

