Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice
- PMID: 18802443
- PMCID: PMC2760737
- DOI: 10.1038/nri2416
Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice
Abstract
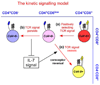
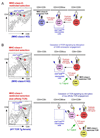
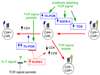
Following successful gene rearrangement at alphabeta T-cell receptor (TCR) loci, developing thymocytes express both CD4 and CD8 co-receptors and undergo a life-or-death selection event, which is known as positive selection, to identify cells that express TCRs with potentially useful ligand specificities. Positively selected thymocytes must then differentiate into either CD4(+) helper T cells or CD8(+) cytotoxic T cells, a crucial decision known as CD4/CD8-lineage choice. In this Review, we summarize recent advances in our understanding of the cellular and molecular events involved in lineage-fate decision and discuss them in the context of the major models of CD4/CD8-lineage choice.
Figures



References
-
- Chong MM, et al. Suppressor of cytokine signaling-1 is a critical regulator of interleukin-7-dependent CD8+ T cell differentiation. Immunity. 2003;18:475–487. - PubMed
-
- Doyle C, Strominger JL. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987;330:256–259. - PubMed
-
- Norment AM, Salter RD, Parham P, Engelhard VH, Littman DR. Cell-cell adhesion mediated by CD8 and MHC class I molecules. Nature. 1988;336:79–81. - PubMed
-
- Shaw AS, et al. The lck tyrosine protein kinase interacts with the cytoplasmic tail of the CD4 glycoprotein through its unique amino-terminal domain. Cell. 1989;59:627–636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

