Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis
- PMID: 18802454
- PMCID: PMC2527686
- DOI: 10.1371/journal.pgen.1000193
Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis
Abstract
The TAR DNA-binding protein 43 (TDP-43) has been identified as the major disease protein in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with ubiquitin inclusions (FTLD-U), defining a novel class of neurodegenerative conditions: the TDP-43 proteinopathies. The first pathogenic mutations in the gene encoding TDP-43 (TARDBP) were recently reported in familial and sporadic ALS patients, supporting a direct role for TDP-43 in neurodegeneration. In this study, we report the identification and functional analyses of two novel and one known mutation in TARDBP that we identified as a result of extensive mutation analyses in a cohort of 296 patients with variable neurodegenerative diseases associated with TDP-43 histopathology. Three different heterozygous missense mutations in exon 6 of TARDBP (p.M337V, p.N345K, and p.I383V) were identified in the analysis of 92 familial ALS patients (3.3%), while no mutations were detected in 24 patients with sporadic ALS or 180 patients with other TDP-43-positive neurodegenerative diseases. The presence of p.M337V, p.N345K, and p.I383V was excluded in 825 controls and 652 additional sporadic ALS patients. All three mutations affect highly conserved amino acid residues in the C-terminal part of TDP-43 known to be involved in protein-protein interactions. Biochemical analysis of TDP-43 in ALS patient cell lines revealed a substantial increase in caspase cleaved fragments, including the approximately 25 kDa fragment, compared to control cell lines. Our findings support TARDBP mutations as a cause of ALS. Based on the specific C-terminal location of the mutations and the accumulation of a smaller C-terminal fragment, we speculate that TARDBP mutations may cause a toxic gain of function through novel protein interactions or intracellular accumulation of TDP-43 fragments leading to apoptosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
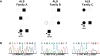



References
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. - PubMed
-
- Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown S, Du Plessis D, et al. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol. 2007;113:521–533. - PubMed
-
- Kwong LK, Uryu K, Trojanowski JQ, Lee VM. TDP-43 proteinopathies: neurodegenerative protein misfolding diseases without amyloidosis. Neurosignals. 2008;16:41–51. - PubMed
-
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS42759-04S1/NS/NINDS NIH HHS/United States
- P50 NS040256/NS/NINDS NIH HHS/United States
- P30 AG13854/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- P50 NS 40256/NS/NINDS NIH HHS/United States
- P01 AG17216/AG/NIA NIH HHS/United States
- R01 AG015866/AG/NIA NIH HHS/United States
- P01 NS040256/NS/NINDS NIH HHS/United States
- P50 AG25711/AG/NIA NIH HHS/United States
- P01 AG003949/AG/NIA NIH HHS/United States
- U01 AG006786/AG/NIA NIH HHS/United States
- R01 NS042759/NS/NINDS NIH HHS/United States
- P30 AG013854/AG/NIA NIH HHS/United States
- P50 AG16574/AG/NIA NIH HHS/United States
- P01 AG03949/AG/NIA NIH HHS/United States
- G0500289/MRC_/Medical Research Council/United Kingdom
- R01 NS42759/NS/NINDS NIH HHS/United States
- U01 AG06786/AG/NIA NIH HHS/United States
- NS40256/NS/NINDS NIH HHS/United States
- AG19610-01/AG/NIA NIH HHS/United States
- P50 AG025711/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- R01 AG026251/AG/NIA NIH HHS/United States
- P01 AG017216/AG/NIA NIH HHS/United States
- R01 AG026251-01/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

