CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope
- PMID: 18802479
- PMCID: PMC2542849
- DOI: 10.1172/JCI35449
CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope
Erratum in
- J Clin Invest. 2009 Sep;119(9):2844. Unger, Wendy [corrected to Unger, Wendy W]
Abstract
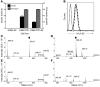
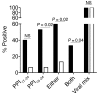
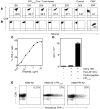
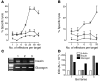
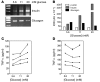
The final pathway of beta cell destruction leading to insulin deficiency, hyperglycemia, and clinical type 1 diabetes is unknown. Here we show that circulating CTLs can kill beta cells via recognition of a glucose-regulated epitope. First, we identified 2 naturally processed epitopes from the human preproinsulin signal peptide by elution from HLA-A2 (specifically, the protein encoded by the A*0201 allele) molecules. Processing of these was unconventional, requiring neither the proteasome nor transporter associated with processing (TAP). However, both epitopes were major targets for circulating effector CD8+ T cells from HLA-A2+ patients with type 1 diabetes. Moreover, cloned preproinsulin signal peptide-specific CD8+ T cells killed human beta cells in vitro. Critically, at high glucose concentration, beta cell presentation of preproinsulin signal epitope increased, as did CTL killing. This study provides direct evidence that autoreactive CTLs are present in the circulation of patients with type 1 diabetes and that they can kill human beta cells. These results also identify a mechanism of self-antigen presentation that is under pathophysiological regulation and could expose insulin-producing beta cells to increasing cytotoxicity at the later stages of the development of clinical diabetes. Our findings suggest that autoreactive CTLs are important targets for immune-based interventions in type 1 diabetes and argue for early, aggressive insulin therapy to preserve remaining beta cells.
Figures



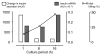
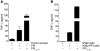
Comment in
-
Novel epitope begets a novel pathway in type 1 diabetes progression.J Clin Invest. 2008 Oct;118(10):3268-71. doi: 10.1172/JCI37125. J Clin Invest. 2008. PMID: 18802485 Free PMC article.
References
-
- Cnop M., et al. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl. 2):S97–S107. - PubMed
-
- Bottazzo G.F., et al. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N. Engl. J. Med. 1985;313:353–360. - PubMed
-
- Somoza N., et al. Pancreas in recent onset insulin-dependent diabetes mellitus. Changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor V beta usage, and cytokine profile. J. Immunol. 1994;153:1360–1377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

