Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals
- PMID: 18802496
- PMCID: PMC2542885
- DOI: 10.1172/JCI32460
Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals
Erratum in
- J Clin Invest. 2012 Nov 1;122(11):4301
Abstract
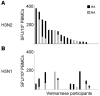
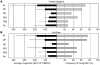
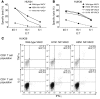
The threat of avian influenza A (H5N1) infection in humans remains a global health concern. Current influenza vaccines stimulate antibody responses against the surface glycoproteins but are ineffective against strains that have undergone significant antigenic variation. An alternative approach is to stimulate pre-existing memory T cells established by seasonal human influenza A infection that could cross-react with H5N1 by targeting highly conserved internal proteins. To determine how common cross-reactive T cells are, we performed a comprehensive ex vivo analysis of cross-reactive CD4+ and CD8+ memory T cell responses to overlapping peptides spanning the full proteome of influenza A/Viet Nam/CL26/2005 (H5N1) and influenza A/New York/232/2004 (H3N2) in healthy individuals from the United Kingdom and Viet Nam. Memory CD4+ and CD8+ T cells isolated from the majority of participants exhibited human influenza-specific responses and showed cross-recognition of at least one H5N1 internal protein. Participant CD4+ and CD8+ T cells recognized multiple synthesized influenza peptides, including peptides from the H5N1 strain. Matrix protein 1 (M1) and nucleoprotein (NP) were the immunodominant targets of cross-recognition. In addition, cross-reactive CD4+ and CD8+ T cells recognized target cells infected with recombinant vaccinia viruses expressing either H5N1 M1 or NP. Thus, vaccine formulas inducing heterosubtypic T cell-mediated immunity may confer broad protection against avian and human influenza A viruses.
Figures
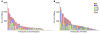


Comment in
-
Toward a broadly protective influenza vaccine.J Clin Invest. 2008 Oct;118(10):3273-5. doi: 10.1172/JCI37232. J Clin Invest. 2008. PMID: 18802488 Free PMC article.
References
-
- WHO. 2008. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008... .
-
- WHO. 2007. Antigenic and genetic characteristics of H5N1 viruses and candidate H5N1 vaccine viruses developed for potential use as pre-pandemic vaccines. http://www.who.int/csr/disease/avian_influenza/guidelines/recommendation.... - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

