High expression of PGE2 enzymatic pathways in cervical (pre)neoplastic lesions and functional consequences for antigen-presenting cells
- PMID: 18802697
- PMCID: PMC11030941
- DOI: 10.1007/s00262-008-0584-4
High expression of PGE2 enzymatic pathways in cervical (pre)neoplastic lesions and functional consequences for antigen-presenting cells
Abstract
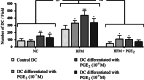
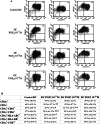
Although human papillomavirus (HPV) DNA is detected in the majority of squamous intraepithelial lesions (SIL) and carcinoma (SCC) of the uterine cervix, the persistence or progression of cervical lesions suggest that viral antigens are not adequately presented to the immune system. This hypothesis is reinforced by the observation that most SIL show quantitative and functional alterations of Langerhans cells (LC). The aim of this study was to determine whether prostaglandins (PG) may affect LC density in the cervical (pre)neoplastic epithelium. We first demonstrated that the epithelial expression of PGE(2) enzymatic pathways, including cyclooxygenase-2 (COX-2) and microsomal prostaglandin E synthase 1 (mPGES-1), is higher in SIL and SCC compared to the normal exocervical epithelium and inversely correlated to the density of CD1a-positive LC. By using cell migration assays, we next showed that the motility of immature dendritic cells (DC) and DC partially differentiated in vitro in the presence of PGE(2) are differentially affected by PGE(2). Immature DC had a lower ability to migrate in the presence of PGE(2) compared to DC generated in vitro in the presence of PGE(2). Finally, we showed that PGE(2) induced a cytokine production profile and phenotypical features of tolerogenic DC, suggesting that the altered expression of PGE(2) enzymatic pathways may promote the cervical carcinogenesis by favouring (pre)cancer immunotolerance.
Figures






Similar articles
-
MIP3 alpha stimulates the migration of Langerhans cells in models of human papillomavirus (HPV)-associated (pre)neoplastic epithelium.Cancer Immunol Immunother. 2007 Jul;56(7):1087-96. doi: 10.1007/s00262-006-0255-2. Epub 2006 Dec 5. Cancer Immunol Immunother. 2007. PMID: 17146629 Free PMC article.
-
Defensins induce the recruitment of dendritic cells in cervical human papillomavirus-associated (pre)neoplastic lesions formed in vitro and transplanted in vivo.FASEB J. 2007 Sep;21(11):2765-75. doi: 10.1096/fj.06-7646com. Epub 2007 Apr 30. FASEB J. 2007. PMID: 17470569
-
Influence of the mucosal epithelium microenvironment on Langerhans cells: implications for the development of squamous intraepithelial lesions of the cervix.Int J Cancer. 2002 Feb 10;97(5):654-9. doi: 10.1002/ijc.10084. Int J Cancer. 2002. PMID: 11807793
-
Correlation of T-helper secretory differentiation and types of antigen-presenting cells in squamous intraepithelial lesions of the uterine cervix.J Pathol. 1998 Mar;184(3):283-90. doi: 10.1002/(SICI)1096-9896(199803)184:3<283::AID-PATH25>3.0.CO;2-K. J Pathol. 1998. PMID: 9614381
-
Decreased synthesis and expression of TGF-beta1, beta2, and beta3 in epithelium of HPV 16-positive cervical precancer: a study by microdissection, quantitative RT-PCR, and immunocytochemistry.J Pathol. 2000 Dec;192(4):494-501. doi: 10.1002/1096-9896(200012)192:4<494::AID-PATH760>3.0.CO;2-W. J Pathol. 2000. PMID: 11113867
Cited by
-
Artesunate induces apoptosis of bladder cancer cells by miR-16 regulation of COX-2 expression.Int J Mol Sci. 2014 Aug 15;15(8):14298-312. doi: 10.3390/ijms150814298. Int J Mol Sci. 2014. PMID: 25196524 Free PMC article.
-
Deciphering the Multifactorial Susceptibility of Mucosal Junction Cells to HPV Infection and Related Carcinogenesis.Viruses. 2017 Apr 20;9(4):85. doi: 10.3390/v9040085. Viruses. 2017. PMID: 28425968 Free PMC article. Review.
-
Exogenous control of the expression of Group I CD1 molecules competent for presentation of microbial nonpeptide antigens to human T lymphocytes.Clin Dev Immunol. 2011;2011:790460. doi: 10.1155/2011/790460. Epub 2011 Mar 22. Clin Dev Immunol. 2011. PMID: 21603161 Free PMC article. Review.
-
mPGES-1 as a target for cancer suppression: A comprehensive invited review "Phospholipase A2 and lipid mediators".Biochimie. 2010 Jun;92(6):660-4. doi: 10.1016/j.biochi.2010.02.006. Epub 2010 Feb 13. Biochimie. 2010. PMID: 20159031 Free PMC article. Review.
-
Activation of the interleukin-32 pro-inflammatory pathway in response to human papillomavirus infection and over-expression of interleukin-32 controls the expression of the human papillomavirus oncogene.Immunology. 2011 Mar;132(3):410-20. doi: 10.1111/j.1365-2567.2010.03377.x. Epub 2011 Jan 5. Immunology. 2011. PMID: 21208204 Free PMC article.
References
-
- Akasaki Y, Liu G, Chung NH, et al. Induction of a CD4 + T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. J Immunol. 2004;173:4352–4359. - PubMed
-
- Al-Saleh W, Giannini SL, Jacobs N, et al. Correlation of T-helper secretory differentiation and types of antigen-presenting cells in squamous intraepithelial lesions of the uterine cervix. J Pathol. 1998;184:283–290. doi: 10.1002/(SICI)1096-9896(199803)184:3<283::AID-PATH25>3.0.CO;2-K. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

