Calcium influx mechanisms underlying calcium oscillations in rat hepatocytes
- PMID: 18802964
- PMCID: PMC2808042
- DOI: 10.1002/hep.22461
Calcium influx mechanisms underlying calcium oscillations in rat hepatocytes
Abstract
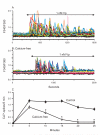
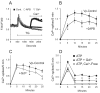
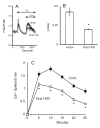
The process of capacitative or store-operated Ca(2+) entry has been extensively investigated, and recently two major molecular players in this process have been described. Stromal interacting molecule (STIM) 1 acts as a sensor for the level of Ca(2+) stored in the endoplasmic reticulum, and Orai proteins constitute pore-forming subunits of the store-operated channels. Store-operated Ca(2+) entry is readily demonstrated with protocols that provide extensive Ca(2+) store depletion; however, the role of store-operated entry with modest and more physiological cell stimuli is less certain. Recent studies have addressed this question in cell lines; however, the role of store-operated entry during physiological activation of primary cells has not been extensively investigated, and there is little or no information on the roles of STIM and Orai proteins in primary cells. Also, the nature of the Ca(2+) influx mechanism with hormone activation of hepatocytes is controversial. Hepatocytes respond to physiological levels of glycogenolytic hormones with well-characterized intracellular Ca(2+) oscillations. In the current study, we have used both pharmacological tools and RNA interference (RNAi)-based techniques to investigate the role of store-operated channels in the maintenance of hormone-induced Ca(2+) oscillations in rat hepatocytes. Pharmacological inhibitors of store-operated channels blocked thapsigargin-induced Ca(2+) entry but only partially reduced the frequency of Ca(2+) oscillations. Similarly, RNAi knockdown of STIM1 or Orai1 substantially reduced thapsigargin-induced calcium entry, and more modestly diminished the frequency of vasopressin-induced oscillations.
Conclusion: Our findings establish that store-operated Ca(2+) entry plays a role in the maintenance of agonist-induced oscillations in primary rat hepatocytes but indicate that other agonist-induced entry mechanisms must be involved to a significant extent.
Figures





References
-
- Woods NM, Cuthbertson KS, Cobbold PH. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986;319:600–602. - PubMed
-
- Rooney TA, Sass EJ, Thomas AP. Characterization of cytosolic calcium oscillations induced by phenylephrine and vasopressin in single fura-2-loaded hepatocytes. J Biol Chem. 1989;264:17131–17141. - PubMed
-
- Robb-Gaspers LD, Thomas AP. Coordination of Ca2+ signaling by intercellular propagation of Ca2+ waves in the intact liver. J Biol Chem. 1995;270:8102–8107. - PubMed
-
- Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
