Low-density lipoprotein receptors regulate microglial inflammation through c-Jun N-terminal kinase
- PMID: 18803301
- PMCID: PMC2628955
- DOI: 10.1002/glia.20772
Low-density lipoprotein receptors regulate microglial inflammation through c-Jun N-terminal kinase
Abstract
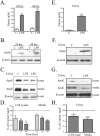
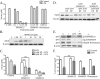
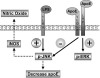
Apolipoprotein E (apoE) has been implicated in modulating the central nervous system (CNS) inflammatory response. However, the molecular mechanisms involved in apoE-dependent immunomodulation are poorly understood. We hypothesize that apoE alters the CNS inflammatory response by signaling via low-density lipoprotein (LDL) receptors in glia. To address this hypothesis, we used a small bioactive peptide formed from the receptor-binding domain of apoE, apoE peptide (EP), to study LDL receptor signaling in microglia. To model glial activation, we treated primary mouse microglia and the microglial cell line BV2 with lipopolysaccharide (LPS) and studied two inflammatory responses: an increase in nitric oxide production (NO) and a decrease in apoE production. We found that treatment of primary microglia and BV2 cells with EP attenuated LPS-induced NO accumulation and apoE reduction in a dose-dependent manner. Using the receptor-associated protein to block ligand binding to members of the LDL receptor family, we found that EP attenuated both of these LPS-induced inflammatory responses via LDL receptors. We studied two intracellular signaling cascades associated with apoE: c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK). LPS induced both ERK and JNK activation, whereas EP induced ERK activation, but drastically reduced JNK activation. Inhibition of JNK with SP600125 reduced LPS-induced NO production and apoE reduction in a dose-dependent manner. Treatment of microglia with suboptimal EP in combination with JNK inhibitor enhanced attenuation of LPS-induced NO production. These data suggest that microglial LDL receptors regulate JNK activation, which is necessary for apoE modulation of the inflammatory response.
Figures



Similar articles
-
LRP1 modulates the microglial immune response via regulation of JNK and NF-κB signaling pathways.J Neuroinflammation. 2016 Dec 8;13(1):304. doi: 10.1186/s12974-016-0772-7. J Neuroinflammation. 2016. PMID: 27931217 Free PMC article.
-
Dexmedetomidine inhibits inducible nitric oxide synthase in lipopolysaccharide-stimulated microglia by suppression of extracellular signal-regulated kinase.Neurol Res. 2015 Mar;37(3):238-45. doi: 10.1179/1743132814Y.0000000426. Epub 2014 Aug 1. Neurol Res. 2015. PMID: 25082544
-
Microglial low-density lipoprotein receptor-related protein 1 modulates c-Jun N-terminal kinase activation.J Neuroimmunol. 2009 Sep 29;214(1-2):25-32. doi: 10.1016/j.jneuroim.2009.06.010. Epub 2009 Jul 7. J Neuroimmunol. 2009. PMID: 19586665 Free PMC article.
-
Suppression of LPS-Induced Inflammation and Cell Migration by Azelastine through Inhibition of JNK/NF-κB Pathway in BV2 Microglial Cells.Int J Mol Sci. 2021 Aug 23;22(16):9061. doi: 10.3390/ijms22169061. Int J Mol Sci. 2021. PMID: 34445767 Free PMC article.
-
Multiple pathways of apolipoprotein E signaling in primary neurons.J Neurochem. 2005 Apr;93(1):145-55. doi: 10.1111/j.1471-4159.2004.03007.x. J Neurochem. 2005. PMID: 15773914
Cited by
-
APOE Genotype Alters Immunoglobulin Subtypes in Knock-In Mice.J Alzheimers Dis. 2015;46(2):365-74. doi: 10.3233/JAD-142184. J Alzheimers Dis. 2015. PMID: 25737044 Free PMC article.
-
Extracellular proteolysis of apolipoprotein E (apoE) by secreted serine neuronal protease.PLoS One. 2014 Mar 27;9(3):e93120. doi: 10.1371/journal.pone.0093120. eCollection 2014. PLoS One. 2014. PMID: 24675880 Free PMC article.
-
JNK3 Overexpression in the Entorhinal Cortex Impacts on the Hippocampus and Induces Cognitive Deficiencies and Tau Misfolding.ACS Chem Neurosci. 2023 Jun 7;14(11):2074-2088. doi: 10.1021/acschemneuro.3c00092. Epub 2023 May 26. ACS Chem Neurosci. 2023. PMID: 37236204 Free PMC article.
-
JNK and NADPH oxidase involved in fluoride-induced oxidative stress in BV-2 microglia cells.Mediators Inflamm. 2013;2013:895975. doi: 10.1155/2013/895975. Epub 2013 Aug 29. Mediators Inflamm. 2013. PMID: 24072958 Free PMC article.
-
Expression and secretion of apoE isoforms in astrocytes and microglia during inflammation.Glia. 2021 Jun;69(6):1478-1493. doi: 10.1002/glia.23974. Epub 2021 Feb 8. Glia. 2021. PMID: 33556209 Free PMC article.
References
-
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406(6797):782–7. - PubMed
-
- Beffert U, Stolt PC, Herz J. Functions of lipoprotein receptors in neurons. J Lipid Res. 2004;45(3):403–9. - PubMed
-
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18(5):1633–41. - PMC - PubMed
-
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27(2-3):229–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

