Anti-tumor activity of erlotinib in the BxPC-3 pancreatic cancer cell line
- PMID: 18803351
- PMCID: PMC2744159
- DOI: 10.3748/wjg.14.5403
Anti-tumor activity of erlotinib in the BxPC-3 pancreatic cancer cell line
Abstract
Aim: To investigate the effect and mechanism of action of erlotinib, an epidermal growth factor receptor (EGFR) small molecule tyrosine kinase inhibitor (TKI), in the human pancreatic cancer cell line BxPC-3 both in vitro and in vivo.
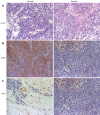
Methods: In vitro, human pancreatic cancer cell line BxPC-3 was exposed to varying concentrations of erlotinib, and its effects on proliferation, cell cycle distribution, apoptosis and the expression of pro- and antiapoptotic factors such as bcl-2, bcl-xl, bax and bak, and the expression of vascular endothelial cell growth factor (VEGF) were measured with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, flow cytometric analysis, terminal deoxynucleotidyl transferase-mediated nick end labeling assay (TUNEL), and reverse transcription-polymerase chain reaction (RT-PCR). Potential effect of erlotinib on angiogenesis was examined by tube formation assay. Tumor growth suppression was observed in xenografted nude mice with pancreatic cancer in vivo. Immunohistochemical (IHC) staining for EGFR and factor VIII-related antigen was undertaken to detect the microvessel density and VEGF expression in tumor tissue in xenograft nude mice.
Results: Erlotinib, as a single agent, repressed BxPC-3 cell growth in a dose-dependent manner, triggered G(1) arrest and induced cell apoptosis, and suppressed capillary formation of endothelium in vitro. Expressions of VEGF were significantly down-regulated at a high concentration of 200 mumol/L, however, the expressions of bcl-2 and bcl-xl were decreased at 50 mumol/L. In vivo, Erlotinib-treated mice demonstrated a reduced tumor volume, weight and microvessel density as compared to the control. IHC staining showed decreased expression of EGFR and RT-PCR had lower VEGF expression in treated mice.
Conclusion: The in vitro and in vivo findings provide evidence that BxPC-3 cells are inhibited with erlotinib treatment. Inhibition of EGFR may be a promising adjuvant chemotherapy strategy in pancreatic cancer treatment.
Figures

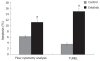



Similar articles
-
Cotargeting of epidermal growth factor receptor and PI3K overcomes PI3K-Akt oncogenic dependence in pancreatic ductal adenocarcinoma.Clin Cancer Res. 2014 Aug 1;20(15):4047-58. doi: 10.1158/1078-0432.CCR-13-3377. Epub 2014 Jun 3. Clin Cancer Res. 2014. PMID: 24895459
-
Simultaneous targeting of the epidermal growth factor receptor and cyclooxygenase-2 pathways for pancreatic cancer therapy.Mol Cancer Ther. 2005 Dec;4(12):1943-51. doi: 10.1158/1535-7163.MCT-05-0065. Mol Cancer Ther. 2005. PMID: 16373709
-
Triple inhibition of EGFR, Met, and VEGF suppresses regrowth of HGF-triggered, erlotinib-resistant lung cancer harboring an EGFR mutation.J Thorac Oncol. 2014 Jun;9(6):775-83. doi: 10.1097/JTO.0000000000000170. J Thorac Oncol. 2014. PMID: 24828661 Free PMC article.
-
Erlotinib in cancer treatment.Ann Oncol. 2007 Jun;18 Suppl 6:vi35-41. doi: 10.1093/annonc/mdm222. Ann Oncol. 2007. PMID: 17591829 Review.
-
Targeting EGFR in pancreatic cancer treatment.Curr Drug Targets. 2012 Jun;13(6):802-10. doi: 10.2174/138945012800564158. Curr Drug Targets. 2012. PMID: 22458527 Review.
Cited by
-
Erlotinib-cisplatin combination inhibits growth and angiogenesis through c-MYC and HIF-1α in EGFR-mutated lung cancer in vitro and in vivo.Neoplasia. 2015 Feb;17(2):190-200. doi: 10.1016/j.neo.2014.12.008. Neoplasia. 2015. PMID: 25748238 Free PMC article.
-
Schedule-dependent cytotoxic synergism of pemetrexed and erlotinib in BXPC-3 and PANC-1 human pancreatic cancer cells.Exp Ther Med. 2011 Sep;2(5):969-975. doi: 10.3892/etm.2011.293. Epub 2011 Jun 22. Exp Ther Med. 2011. PMID: 22977607 Free PMC article.
-
Erlotinib Pretreatment Improves Photodynamic Therapy of Non-Small Cell Lung Carcinoma Xenografts via Multiple Mechanisms.Cancer Res. 2015 Aug 1;75(15):3118-26. doi: 10.1158/0008-5472.CAN-14-3304. Epub 2015 Jun 8. Cancer Res. 2015. PMID: 26054596 Free PMC article.
-
Knockdown delta-5-desaturase promotes the formation of a novel free radical byproduct from COX-catalyzed ω-6 peroxidation to induce apoptosis and sensitize pancreatic cancer cells to chemotherapy drugs.Free Radic Biol Med. 2016 Aug;97:342-350. doi: 10.1016/j.freeradbiomed.2016.06.028. Epub 2016 Jun 28. Free Radic Biol Med. 2016. PMID: 27368132 Free PMC article.
-
Systemic deuteration of SCID mice using the water-isotopologue deuterium oxide (D2 O) inhibits tumor growth in an orthotopic bioluminescent model of human pancreatic ductal adenocarcinoma.Mol Carcinog. 2023 May;62(5):598-612. doi: 10.1002/mc.23509. Epub 2023 Feb 2. Mol Carcinog. 2023. PMID: 36727657 Free PMC article.
References
-
- Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. - PubMed
-
- MacKenzie MJ. Molecular therapy in pancreatic adenocarcinoma. Lancet Oncol. 2004;5:541–549. - PubMed
-
- Matsuda K, Idezawa T, You XJ, Kothari NH, Fan H, Korc M. Multiple mitogenic pathways in pancreatic cancer cells are blocked by a truncated epidermal growth factor receptor. Cancer Res. 2002;62:5611–5617. - PubMed
-
- Solorzano CC, Baker CH, Tsan R, Traxler P, Cohen P, Buchdunger E, Killion JJ, Fidler IJ. Optimization for the blockade of epidermal growth factor receptor signaling for therapy of human pancreatic carcinoma. Clin Cancer Res. 2001;7:2563–2572. - PubMed
-
- Bruns CJ, Solorzano CC, Harbison MT, Ozawa S, Tsan R, Fan D, Abbruzzese J, Traxler P, Buchdunger E, Radinsky R, et al. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–2935. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

