Expeditious chemoenzymatic synthesis of homogeneous N-glycoproteins carrying defined oligosaccharide ligands
- PMID: 18803385
- PMCID: PMC2662341
- DOI: 10.1021/ja805044x
Expeditious chemoenzymatic synthesis of homogeneous N-glycoproteins carrying defined oligosaccharide ligands
Abstract
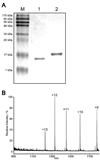
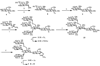
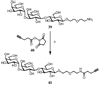
An efficient chemoenzymatic method for the construction of homogeneous N-glycoproteins was described that explores the transglycosylation activity of the endo-beta-N-acetylglucosaminidase from Arthrobacter protophormiae (Endo-A) with synthetic sugar oxazolines as the donor substrates. First, an array of large oligosaccharide oxazolines were synthesized and evaluated as substrates for the Endo-A-catalyzed transglycosylation by use of ribonuclease B as a model system. The experimental results showed that Endo-A could tolerate modifications at the outer mannose residues of the Man3GlcNAc-oxazoline core, thus allowing introduction of large oligosaccharide ligands into a protein and meanwhile preserving the natural, core N-pentasaccharide (Man3GlcNAc2) structure in the resulting glycoprotein upon transglycosylation. In addition to ligands for galectins and mannose-binding lectins, azido functionality could be readily introduced at the N-pentasaccharide (Man3GlcNAc2) core by use of azido-containing Man3GlcNAc oxazoline as the donor substrate. The introduction of azido functionality permits further site-specific modifications of the resulting glycoproteins, as demonstrated by the successful attachment of two copies of alphaGal epitopes to ribonuclease B. This study reveals a broad substrate specificity of Endo-A for transglycosylation, and the chemoenzymatic method described here points to a new avenue for quick access to various homogeneous N-glycoproteins for structure-activity relationship studies and for biomedical applications.
Figures








Similar articles
-
Chemoenzymatic synthesis of neoglycoproteins using transglycosylation with endo-beta-N-acetylglucosaminidase A.Biochem Biophys Res Commun. 2001 Apr 6;282(3):678-82. doi: 10.1006/bbrc.2001.4631. Biochem Biophys Res Commun. 2001. PMID: 11401514
-
Glycosynthases enable a highly efficient chemoenzymatic synthesis of N-glycoproteins carrying intact natural N-glycans.J Am Chem Soc. 2009 Feb 18;131(6):2214-23. doi: 10.1021/ja8074677. J Am Chem Soc. 2009. PMID: 19199609 Free PMC article.
-
Chemoenzymatic synthesis of glycopeptides and glycoproteins through endoglycosidase-catalyzed transglycosylation.Carbohydr Res. 2008 Jul 21;343(10-11):1509-22. doi: 10.1016/j.carres.2008.03.025. Epub 2008 Mar 27. Carbohydr Res. 2008. PMID: 18405887 Free PMC article. Review.
-
Arthrobacter endo-beta-N-acetylglucosaminidase shows transglycosylation activity on complex-type N-glycan oxazolines: one-pot conversion of ribonuclease B to sialylated ribonuclease C.Chembiochem. 2010 Jul 5;11(10):1350-5. doi: 10.1002/cbic.201000242. Chembiochem. 2010. PMID: 20486148 Free PMC article.
-
The ENGases: versatile biocatalysts for the production of homogeneous N-linked glycopeptides and glycoproteins.Chem Soc Rev. 2017 Aug 14;46(16):5128-5146. doi: 10.1039/c6cs00897f. Chem Soc Rev. 2017. PMID: 28681051 Review.
Cited by
-
Synthesis of glycopeptides and glycopeptide conjugates.Org Biomol Chem. 2022 Aug 24;20(33):6487-6507. doi: 10.1039/d2ob00829g. Org Biomol Chem. 2022. PMID: 35903971 Free PMC article. Review.
-
Unusual transglycosylation activity of Flavobacterium meningosepticum endoglycosidases enables convergent chemoenzymatic synthesis of core fucosylated complex N-glycopeptides.Chembiochem. 2011 Apr 11;12(6):932-41. doi: 10.1002/cbic.201000763. Epub 2011 Mar 4. Chembiochem. 2011. PMID: 21374780 Free PMC article.
-
Chemoenzymatic synthesis and Fcγ receptor binding of homogeneous glycoforms of antibody Fc domain. Presence of a bisecting sugar moiety enhances the affinity of Fc to FcγIIIa receptor.J Am Chem Soc. 2011 Nov 23;133(46):18975-91. doi: 10.1021/ja208390n. Epub 2011 Nov 1. J Am Chem Soc. 2011. PMID: 22004528 Free PMC article.
-
Synthetic, Zwitterionic Sp1 Oligosaccharides Adopt a Helical Structure Crucial for Antibody Interaction.ACS Cent Sci. 2019 Aug 28;5(8):1407-1416. doi: 10.1021/acscentsci.9b00454. Epub 2019 Jul 24. ACS Cent Sci. 2019. PMID: 31482123 Free PMC article.
-
Glycosylated analogs of formaecin I and drosocin exhibit differential pattern of antibacterial activity.Glycoconj J. 2011 Dec;28(8-9):537-55. doi: 10.1007/s10719-011-9353-2. Epub 2011 Oct 4. Glycoconj J. 2011. PMID: 21968924
References
-
- Imperiali B, O'Connor SE. Curr. Opin. Chem. Biol. 1999;3:643–649. - PubMed
- Sola RJ, Rodriguez-Martinez JA, Griebenow K. Cell Mol Life Sci. 2007;64:2133–2152. - PMC - PubMed
- Helenius A, Aebi M. Science. 2001;291:2364–2369. - PubMed
- Petrescu AJ, Wormald MR, Dwek RA. Curr. Opin. Struct. Biol. 2006;16:600–607. - PubMed
-
- Varki A. Glycobiology. 1993;3:97–130. - PMC - PubMed
- Dwek RA. Chem. Rev. 1996;96:683–720. - PubMed
- Dwek RA, Butters TD, Platt FM, Zitzmann N. Nat. Rev. Drug. Discov. 2002;1:65–75. - PubMed
- Haltiwanger RS, Lowe JB. Annu. Rev. Biochem. 2004;73:491–537. - PubMed
- Dube DH, Bertozzi CR. Nat. Rev. Drug. Discov. 2005;4:477–488. - PubMed
-
- Koeller KM, Wong CH. Nat. Biotechnol. 2000;18:835–841. - PubMed
- Seitz O. ChemBioChem. 2000;1:214–246. - PubMed
- Herzner H, Reipen T, Schultz M, Kunz H. Chem. Rev. 2000;100:4495–4538. - PubMed
- Hang HC, Bertozzi CR. Acc. Chem. Res. 2001;34:727–736. - PubMed
- Davis BG. Chem. Rev. 2002;102:579–601. - PubMed
- Grogan MJ, Pratt MR, Marcaurelle LA, Bertozzi CR. Annu. Rev. Biochem. 2002;71:593–634. - PubMed
- Wong CH. J. Org. Chem. 2005;70:4219–4225. - PubMed
- Guo Z, Shao N. Med. Res. Rev. 2005;25:655–678. - PubMed
- Pratt MR, Bertozzi CR. Chem. Soc. Rev. 2005;34:58–68. - PubMed
- Liu L, Bennett CS, Wong CH. Chem. Commun. 2006:21–33. - PubMed
- Buskas T, Ingale S, Boons GJ. Glycobiology. 2006;16:113R–136R. - PubMed
- Brik A, Ficht S, Wong CH. Curr. Opin. Chem. Biol. 2006;10:638–644. - PubMed
- Bennett CS, Wong CH. Chem. Soc. Rev. 2007;36:1227–1238. - PubMed
- Wildt S, Gerngross TU. Nat. Rev. Microbiol. 2005;3:119–128. - PubMed
- Yu H, Chen X. Org. Biomol. Chem. 2007;5:865–872. - PMC - PubMed
-
- Warren JD, Miller JS, Keding SJ, Danishefsky SJ. J. Am. Chem. Soc. 2004;126:6576–6578. - PubMed
- Macmillan D, Bertozzi CR. Angew. Chem. Int. Ed. 2004;43:1355–1359. - PubMed
- Hojo H, Matsumoto Y, Nakahara Y, Ito E, Suzuki Y, Suzuki M, Suzuki A. J. Am. Chem. Soc. 2005;127:13720–13725. - PubMed
- Wu B, Chen J, Warren JD, Chen G, Hua Z, Danishefsky SJ. Angew. Chem. Int. Ed. 2006;45:4116–4125. - PubMed
- Brik A, Ficht S, Yang YY, Bennett CS, Wong CH. J. Am. Chem. Soc. 2006;128:15026–15033. - PubMed
- Payne RJ, Ficht S, Tang S, Brik A, Yang YY, Case DA, Wong CH. J. Am. Chem. Soc. 2007;129:13527–13536. - PubMed
- Yamamoto N, Tanabe Y, Okamoto R, Dawson PE, Kajihara Y. J. Am. Chem. Soc. 2008;130:501–510. - PubMed
-
- Witte K, Sears P, Martin R, Wong CH. J. Am. Chem. Soc. 1997;119:2114–2118.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

